Trends in the periodic table Chapter 7 Atomic

Trends in the periodic table Chapter 7

Atomic Radius • Atomic radius is half the distance between two nuclei of an element joined by a single covalent bond

Atomic radius increases down a group • As more energy levels are added the atomic radius increases • Screening effect: innermost electrons “shield” other electrons from the full nuclear charge

Atomic radius decreases across a period • As more protons are added the nuclear charge increases resulting in a greater force of attraction • There is no increase in screening across a period
Ionisation Energy • First ionisation energy is the energy (k. J) required to completely remove the most loosely bound electron from every atom in a mole of a gaseous element in its ground state • Ionisation energy increases across a period and decreases down a group
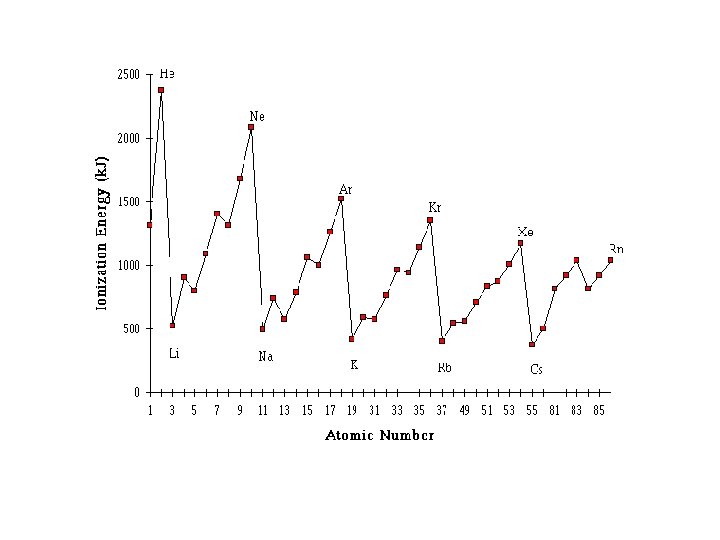

• Atoms with half filled orbitals have greater stability • Therefore it takes more energy to remove an electron from these elements
Electronegativity • The relative attraction of an atom for a shared pair of electrons in a covalent bond • Increases across a period • Decreases down a group

Reactivity of Alkali Metals • Reactivity increases down the group • Ionisation energy and electronegativity decrease down the group • Reaction with oxygen – 2 K + ½O 2 K 2 O • Reaction with water – Na + H 2 O Na. OH + ½H 2

Reactivity of Halogens • Reactivity decreases down the group • Halogens are oxidising agents • In a reaction between halogens, the oxidising agent is higher up the group
- Slides: 10