Trends and health effects of Pb in the

Trends and health effects of Pb in the atmosphere CHM 481 By Albert Baramuli 24 th February 2006

Outline 1. 2. 3. 4. 5. 6. 7. 8. Facts about Lead Production Uses of Lead Tetraethyl Lead-Acid Battery Hazards and Health Effects Actions Against Lead Usage Lead Poisoning

Facts about Lead • Lead has the highest atomic number of all stable elements • Lead has four stable, naturally occurring isotopes: 204 Pb (1. 4%), 206 Pb (24. 1%), 207 Pb (22. 1%), and 208 Pb (52. 4%). • Lead is the only metal in which there is zero Thomson Effect, due to its poor electrical conductivity

Lead Production • Mostly from ore mineral Pb. S (Galena). • Other source include Pb. CO 3 (Cerrusite) and Pb. SO 4 (Anglesite). • Also found naturally in soils at toxic levels • Separated from other sulfides by floatation • Smelting to produce Pb. O • Reduction with coke and electrolysis for pure Pb metal
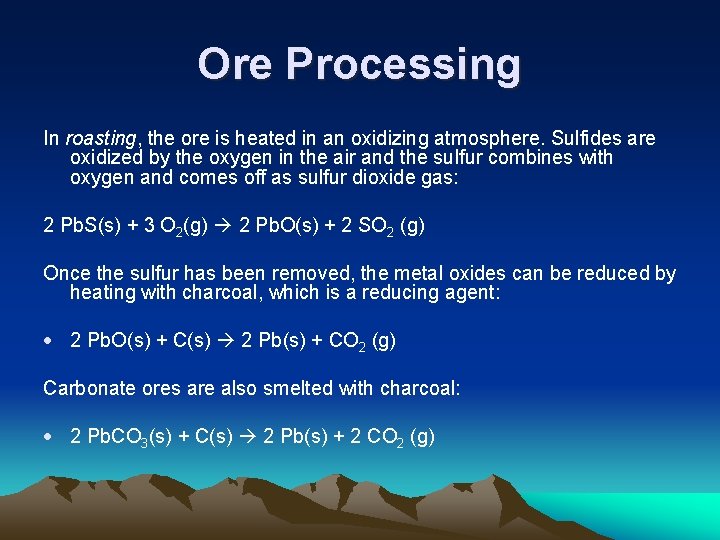
Ore Processing In roasting, the ore is heated in an oxidizing atmosphere. Sulfides are oxidized by the oxygen in the air and the sulfur combines with oxygen and comes off as sulfur dioxide gas: 2 Pb. S(s) + 3 O 2(g) 2 Pb. O(s) + 2 SO 2 (g) Once the sulfur has been removed, the metal oxides can be reduced by heating with charcoal, which is a reducing agent: 2 Pb. O(s) + C(s) 2 Pb(s) + CO 2 (g) Carbonate ores are also smelted with charcoal: 2 Pb. CO 3(s) + C(s) 2 Pb(s) + 2 CO 2 (g)

Uses of Lead • Lead-Acid Battery used extensively for cars. • Lead was used as yellow and red colors pigment for paint and ceramics. • Lead was used for plumbing in Ancient Rome and water mains and service pipes up until the early 1970's. • Lead is used as projectiles for firearms and fishing sinkers • Lead is used in some candles to treat the wick • Lead is used as to make vinyl blinds • Molten lead is used as coolant, usually used in reactors • Lead Glass is comprised of 12 -28% lead. It changes the optical characteristics of the glass and reduces the transmission of radiation. • Tetraethyl Lead was used in leaded fuels • Lead is used in solder for electronics

Use of Lead in Gasoline • Straight gasoline causes premature detonation-- knocking or pinging and poor fuel efficiency. • In 1922, Thomas Midgely (who also invented CFCs) found that if tetraethyl lead, Pb(CH 2 CH 3)4, was put into fuels, particles of lead and lead oxide Pb. O are formed on combustion. • TEL released Pb into the atmosphere during manufacture and from auto engines along with dioxins from additives used to prevent lead deposits from seizing valves. • This helps the petrol to burn more slowly and smoothly, preventing knocking and giving higher Octane ratings • In 1924, Du. Pont and General Motors created the Ethyl Gasoline Corporation to produce and market TEL • The deployment of the catalytic converter (which TEL contaminated and rendered ineffective) reduced TEL use.
Chemistry of Tetraethyl Lead • TEL is produced by reacting ethyl chloride with a sodium-lead alloy. TEL has a very weak carbon-lead bond, and at the temperatures found in internal combustion engines it decomposes into lead and ethyl radicals, propagating the combustion by radical reactions. • When TEL burns, it produces lead and many lead compounds (including lead oxide), which would quickly build up and destroy an engine. • These days, scavengers such as ethylene dibromide and 1, 2 -dichloroetahne are used.

Lead-Acid Battery • Almost all automobile electrical systems • Many portable electrical devices • Battery breaking sites batteries are broken open and the lead electrodes reclaimed. • Contamination of soil and water with lead and acids.

Lead-Acid Battery • On a global scale, 63 percent of all processed lead is used in the manufacturing of batteries • Average exposure levels in children residing near battery plants in developing countries are four times the current level of concern established by the US Center for Disease Control (CDC). • The average worker blood lead levels is approximately twice the recommended level at which workers should be removed from working around lead by the US National Institute for Occupational Safety and Health (NIOSH).



Health Effects • Exposure to lead occurs mainly through inhalation of air and ingestion of lead in food, water, soil, or dust. • Lead accumulates in the blood, bones, and soft tissues and can adversely affect the kidneys, liver, nervous system, and other organs. • Excessive exposure to lead may cause neurological impairments such as seizures, mental retardation, and behavioral disorders. • Lead exposure is associated with damage to the nervous systems of fetuses and young children, resulting in learning deficits and lowered IQ. • Recent studies also show that lead may be a factor in high blood pressure and subsequent heart disease. • Lead is also considered to be particularly harmful for human's ability to reproduce. • The average person has less than 10 micrograms per deciliter, or 100 ppb, of lead in their blood serum. • People who have been exposed to an unusual amount of lead will have lead serum levels higher than 200 ppb—most clinical symptoms of lead poisoning begin at around 100 ppb.

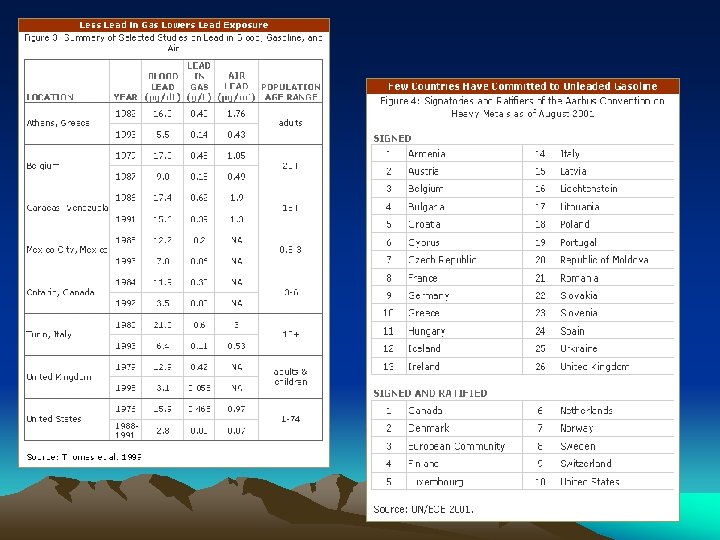
Actions Against Lead Usage • In the US in 1972, the EPA launched an initiative to phase out leaded gasoline, which caused Ethyl Corp. to sue the EPA. The EPA won, so in 1976 the phase out began and was completed by 1986 • Emissions of lead in the US decreased 93 percent over the 21 -year period 1982– 2002. • The Aarhus Convention protocol were signed by 33 governments and the European Community on June 24, 1998. Around 30 countries also endorsed a strategy to phase-out lead in petrol by 2011 at the latest. Most EU countries said they would wipe out leaded petrol by 2005. • Today, industrial processes, primarily metals processing, are the major source of lead emissions to the atmosphere
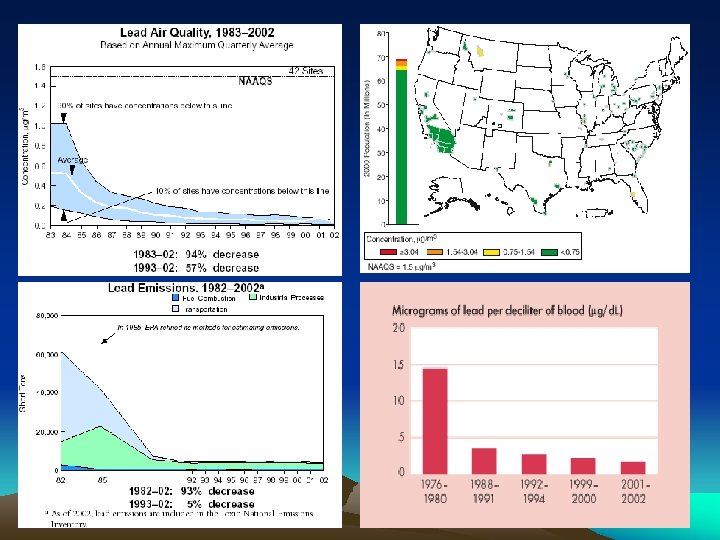
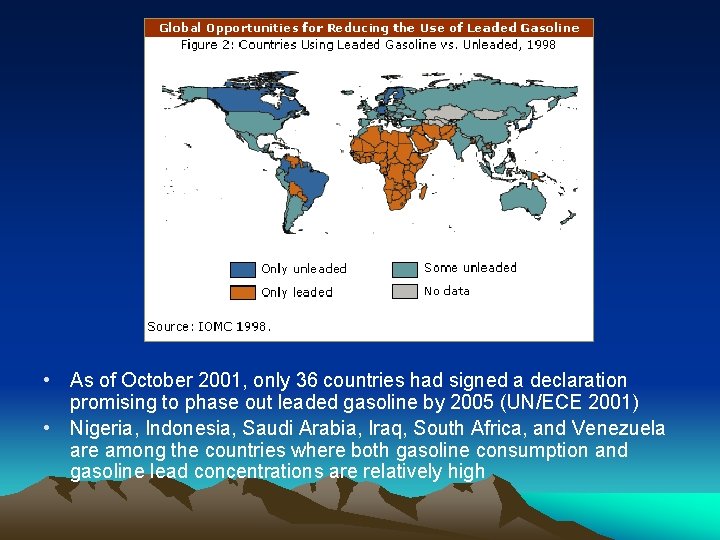
• As of October 2001, only 36 countries had signed a declaration promising to phase out leaded gasoline by 2005 (UN/ECE 2001) • Nigeria, Indonesia, Saudi Arabia, Iraq, South Africa, and Venezuela are among the countries where both gasoline consumption and gasoline lead concentrations are relatively high

Lead Poisoning • The toxicity of Lead comes from its ability to mimic other biologically important metals, the most notable of which are calcium, iron and zinc. • Lead is able to bind to and interact with the same proteins and molecules as these metals, but after displacement, those molecules function differently and fail to carry out the same reactions, such as in producing enzymes. • Most lead poisoning symptoms occur by interfering with an essential enzyme, Delta-aminolevulinic acid dehydratase, or ALAD. • ALAD is a zinc-binding protein which is important in the biosynthesis of heme, the cofactor found in hemoglobin. • Genetic mutations of ALAD cause the disease porphyria.

Lead Poisoning • Mostly happened to children • In developing countries, most cases in due to high leaded gasoline that is still used • In the US, most common way that children get lead poisoned is through lead dust in the home – old paint containing lead • Most fishing sinkers are solid lead. Keep lead fishing sinkers and tackle boxes out the reach of young children. • Some dishes and clay cookware contain high levels of lead in the glaze or decoration • Imported vinyl (plastic) blinds manufactured before 1996 were made with lead. • Due to its sweet taste, Pb is also used as sweetener in candies
- Slides: 20