Trends Across The Periodic Table Periodic Table Periodic

Trends Across The Periodic Table

Periodic Table Periodic table is an organized chart of elements �Organized by the number of protons in the nucleus (represented by the atomic number) �Each atom element is made up of one type of

Periodic Table Four of the elements on the periodic table make up the largest portion of solid Earth, living matter, the oceans, and the atmosphere: Carbon Hydrogen Oxygen Nitrogen “CHON”
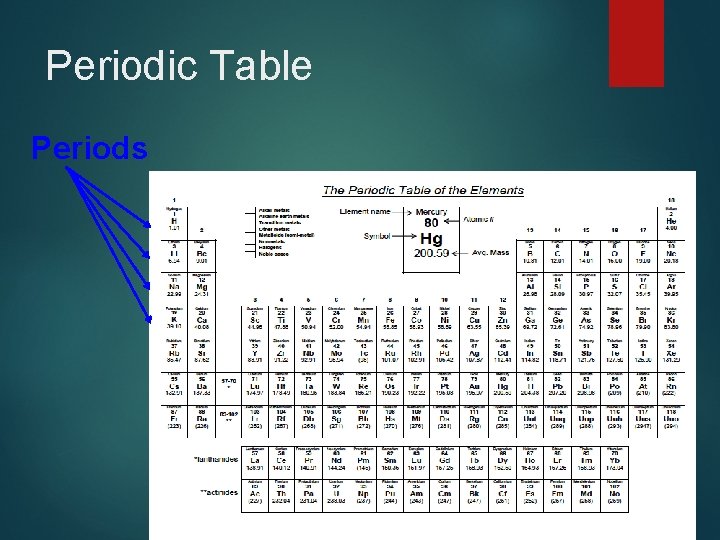
Periodic Table Periods

Periodic Table: Periods Horizontal rows that run left to right across the periodic table �The lanthanides and actinides (rows at the very bottom) actually fit in periods 6 & 7 �The period identifies how many electron shells an atom has �The physical size of the atoms decreases across the period from left to right
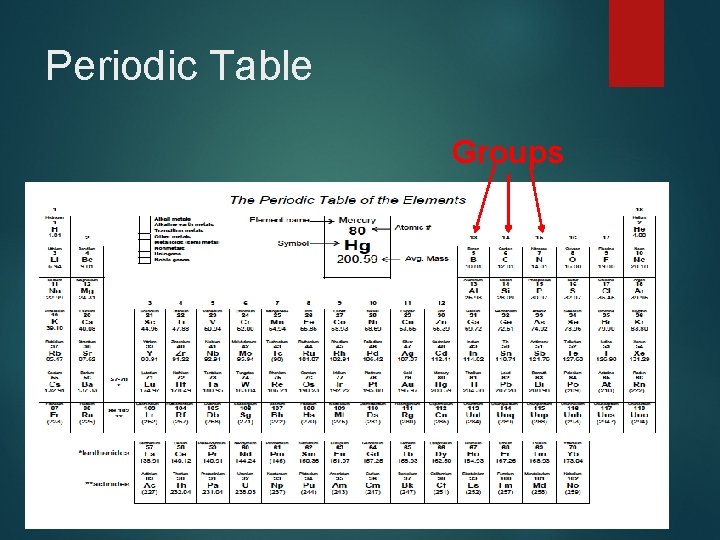
Periodic Table Groups

Periodic Table: Groups Numbered columns that run from top to bottom �Elements in the same groups tend to have similar physical and chemical properties �These groups are sometimes referred to as families, but more than one group can be in the same family

Group: Hydrogen sits in group 1, but belongs to its own family…all alone One valence electron in its outer shell
Group: Alkali Metals Group 1 Soft, silvery-white metals with low melting points One valence electron in its outer shell So reactive that they will burn skin React violently with water Almost never found in their pure forms in nature

Group: Alkaline Earth Metals Group 2 Soft, silvery-white with high melting points and densities Two valence electrons in its outer shell Reactive but can be handled by humans Never found in their pure forms in nature
Groups: Transition Metals Groups 3 -12 Hard, shiny, and strong metals Most of them have high melting and boiling points Good conductors of heat and electricity All have 1 or 2 valence electrons in its outer shell

Group: Boron Group 13 Three valence electrons in its outer shell

Groups: Carbon Group 14 Four valence electrons in its outer shell

Groups: Nitrogen Group 15 Five valence electrons in its outer shell
Groups: Oxygen Group 16 Six valence electrons in its outer shell Wants to react with elements from group 2
Groups: Halogens Group 17 Family of poisonous nonmetals Very reactive and never found in their pure forms in nature Seven valence electrons in its outer shell Wants to react with elements from group 1

Groups: Noble Gases Group 18 Colorless, tasteless, and odorless gases Extremely nonreactive Eight valence electrons in its outer shell

Groups: Lanthanides Found at the bottom of the periodic table Soft, silvery metal Reactive and will burn in oxygen or air Will produce a spark when struck

Groups: Actinides Found at the very bottom of the periodic table Silvery metals Radioactive (emits radiation energy that can damage living tissue)
- Slides: 19