Treatment of Perimenopause and Menopause PPS 946 Dr


































- Slides: 50

Treatment of Perimenopause and Menopause PPS 946 Dr Piascik 1/14/16

Objectives At the end of this session, students should be able to: • Define menopause and perimenopause. • Summarize current therapies and the usefulness of each therapy. • Discuss risks/benefits of HRT based on WHI and subsequent data. • Discuss current recommendations on use of HRT in post-menopausal women. • Discuss usefulness of non-pharmacological options for treatment of menopause.

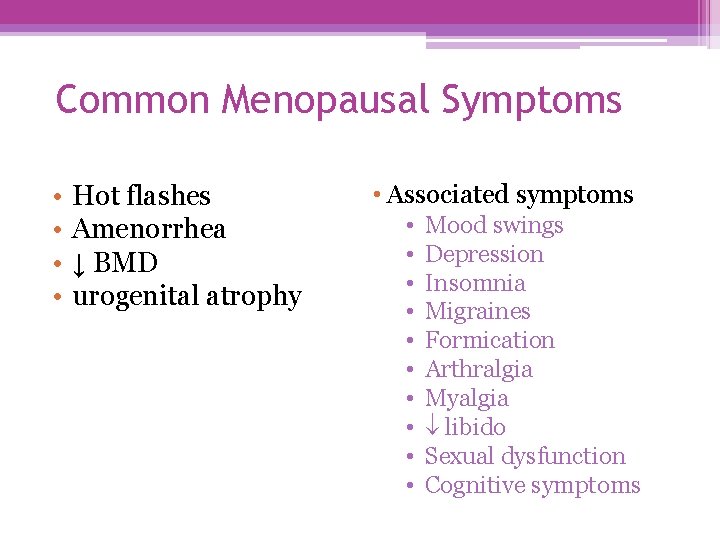
Common Menopausal Symptoms • • Hot flashes Amenorrhea ↓ BMD urogenital atrophy • Associated symptoms • • • Mood swings Depression Insomnia Migraines Formication Arthralgia Myalgia libido Sexual dysfunction Cognitive symptoms

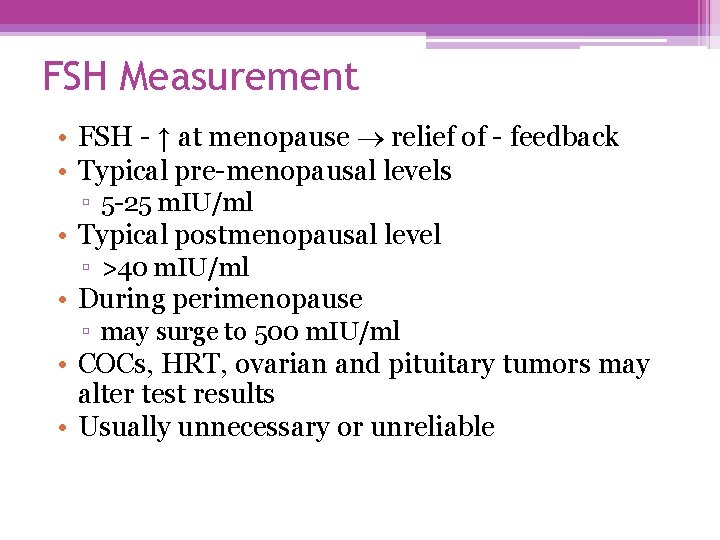
FSH Measurement • FSH - ↑ at menopause relief of - feedback • Typical pre-menopausal levels ▫ 5 -25 m. IU/ml • Typical postmenopausal level ▫ >40 m. IU/ml • During perimenopause ▫ may surge to 500 m. IU/ml • COCs, HRT, ovarian and pituitary tumors may alter test results • Usually unnecessary or unreliable

Goals of Therapy • Relieve menopausal symptoms • Enhance quality of life • Reduce morbidity/mortality associated with sex-hormone deficiency ▫ Bone loss, cardiovascular disease, and diabetes

Therapeutic Treatment Options • Lifestyle modifications • Hormonal therapies ▫ Traditional agents ▫ Bioidentical hormone therapy (BHRT) • Symptomatic relief • Alternative products

Lifestyle Modification • • Smoking cessation Decrease caffeine and alcohol intake Increase exercise For vasomotor symptoms ▫ Dress in layers ▫ Avoid hot and spicy foods, alcohol and caffeine ▫ deep breathing/stress reduction techniques, meditation • OTC vaginal lubricants and moisturizers • Alternative products – discussed later

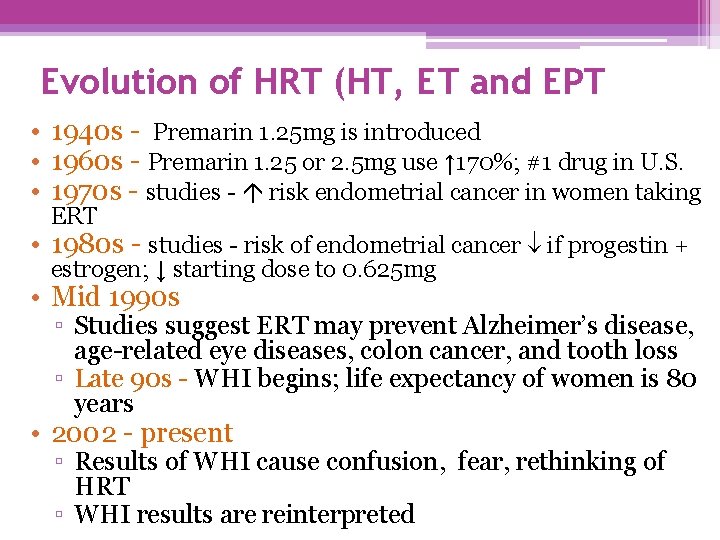
Evolution of HRT (HT, ET and EPT • 1940 s - Premarin 1. 25 mg is introduced • 1960 s - Premarin 1. 25 or 2. 5 mg use ↑ 170%; #1 drug in U. S. • 1970 s - studies - risk endometrial cancer in women taking ERT • 1980 s - studies - risk of endometrial cancer if progestin + estrogen; ↓ starting dose to 0. 625 mg • Mid 1990 s ▫ Studies suggest ERT may prevent Alzheimer’s disease, age-related eye diseases, colon cancer, and tooth loss ▫ Late 90 s - WHI begins; life expectancy of women is 80 years • 2002 - present ▫ Results of WHI cause confusion, fear, rethinking of HRT ▫ WHI results are reinterpreted

Women’s Health Initiative (WHI) • Purpose - define risks & benefits of interventions to heart disease, breast and colorectal cancer, and osteoporotic fractures in postmenopausal women • Conducted at 40 centers; multi-arm study; intended to run from 1997 -2005 • HRT arm terminated in 2002 ▫ due to in cases of breast cancer • ERT arm terminated in 2004 ▫ due to risk of stroke

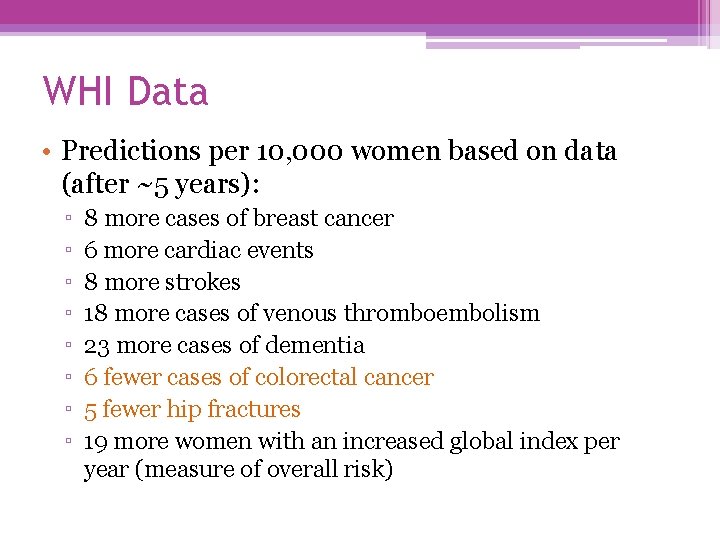
WHI Data • Predictions per 10, 000 women based on data (after ~5 years): ▫ ▫ ▫ ▫ 8 more cases of breast cancer 6 more cardiac events 8 more strokes 18 more cases of venous thromboembolism 23 more cases of dementia 6 fewer cases of colorectal cancer 5 fewer hip fractures 19 more women with an increased global index per year (measure of overall risk)

Qualifications for data from WHI • • study population ~ 63 yrs 1: 6 patients were <5 yrs of menopause Patients primarily asymptomatic Only products used were Prempro 0. 625/2. 5 mg and Premarin 0. 625 mg • Would results be similar in women who are newly diagnosed, symptomatic, using lower doses, using other estrogens and progestins, receiving a different dosage form?

*JAMA 2002 5 years post- WHI report* • 65% of women stopped HRT in 2002 • 1 in 4 women subsequently returned to therapy – why? ▫ Still offers best relief of symptoms ▫ Willing to take risks to get benefits ▫ Follow-up reports of WHI, uncertainty of what results mean

2013 Recommendations for HT* • most effective treatment for vasomotor symptoms at any age • benefits - more likely to outweigh risks for symptomatic women <60 years or within 10 years after menopause • MHT - effective and appropriate to prevent osteoporosis-related fractures in at-risk women <60 yrs or within 10 yrs of menopause • Standard-dose estrogen-alone HT may CHD and all-cause mortality in women <60 yrs and within 10 yrs of menopause • Estrogen plus progestogen HT show similar trend for mortality but in most RCTs, no significant or in CHD has been found • Local low-dose estrogen therapy - preferred for women whose symptoms are limited to vaginal dryness or associated discomfort with intercourse • Estrogen - single systemic agent is appropriate in women after hysterectomy; added progestogen is required in the presence of a uterus 2013 consensus statement, CLIMACTERIC 2013; 16: 203– 204 ACOG – management of Menopausal Symptoms. Practice Bulletin. Vol 123, No. 1, 1/14

2013 Recommendations for HT* • HT - individual decision in terms of QOL, health priorities, and risk factors such as age, time since menopause and risk of VTE, stroke, ischemic heart disease and breast cancer • risk of VTE and ischemic stroke s with oral HT (absolute risk is rare <60 yrs) • Observational studies point to a risk with transdermal therapy • risk of breast cancer in women >50 yrs associated with HT is a complex issue. risk of breast cancer - primarily associated with addition of progestin to estrogen therapy and duration of use. • Risk of breast cancer attributable to HT is small and risk s after treatment is stopped 2013 consensus statement, CLIMACTERIC 2013; 16: 203– 204 ACOG – management of Menopausal Symptoms. Practice Bulletin. Vol 123, No. 1, 1/14

2013 Recommendations for HT* • Dose/duration of HT should be consistent with goals and safety issues and should be individualized • In women with premature ovarian insufficiency, systemic HT is recommended at least until the average of the natural menopause • use of BHT therapy is not recommended • Current safety data do not support use of MHT in breast cancer survivors • SSRIs, SSNRIs, clonidine, and gabapentin are effective alternatives for treatment of vasomotor symptoms ▫ Paroxetine approved • ospemifene approved for treating moderate-to-severe dyspareunia 2013 consensus statement, CLIMACTERIC 2013; 16: 203– 204 ACOG – management of Menopausal Symptoms. Practice Bulletin. Vol 123, No. 1, 1/14

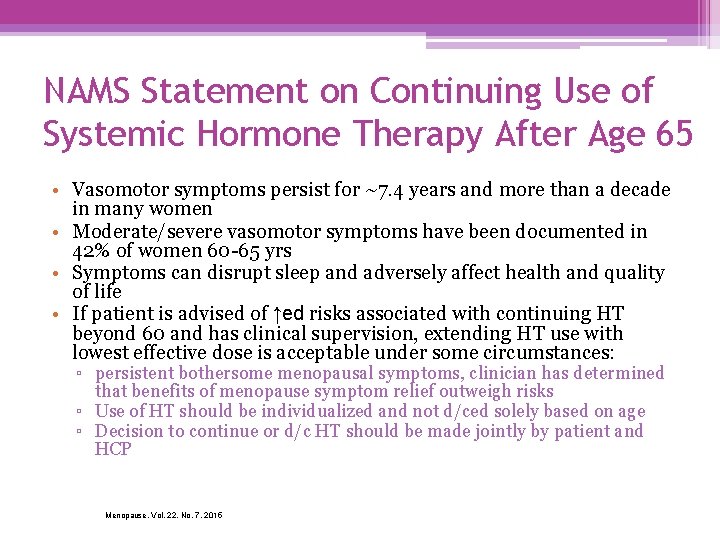
NAMS Statement on Continuing Use of Systemic Hormone Therapy After Age 65 • Vasomotor symptoms persist for ~7. 4 years and more than a decade in many women • Moderate/severe vasomotor symptoms have been documented in 42% of women 60 -65 yrs • Symptoms can disrupt sleep and adversely affect health and quality of life • If patient is advised of ↑ed risks associated with continuing HT beyond 60 and has clinical supervision, extending HT use with lowest effective dose is acceptable under some circumstances: ▫ persistent bothersome menopausal symptoms, clinician has determined that benefits of menopause symptom relief outweigh risks ▫ Use of HT should be individualized and not d/ced solely based on age ▫ Decision to continue or d/c HT should be made jointly by patient and HCP Menopause, Vol. 22, No. 7, 2015

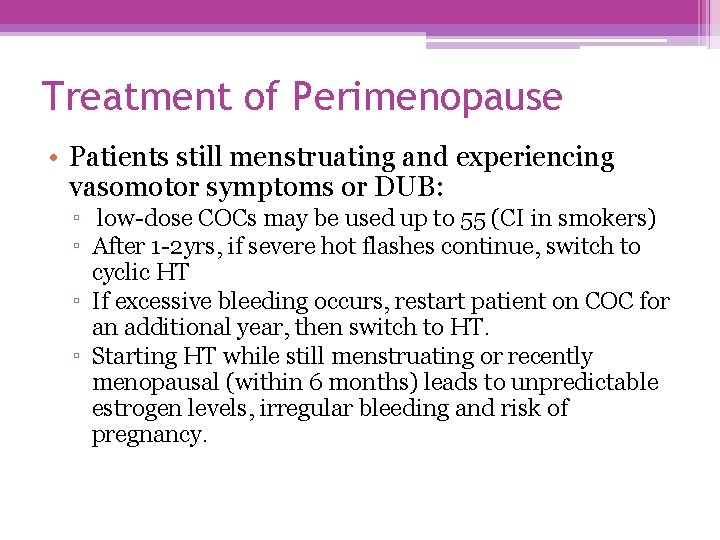
Treatment of Perimenopause • Patients still menstruating and experiencing vasomotor symptoms or DUB: ▫ low-dose COCs may be used up to 55 (CI in smokers) ▫ After 1 -2 yrs, if severe hot flashes continue, switch to cyclic HT ▫ If excessive bleeding occurs, restart patient on COC for an additional year, then switch to HT. ▫ Starting HT while still menstruating or recently menopausal (within 6 months) leads to unpredictable estrogen levels, irregular bleeding and risk of pregnancy.

Pharmacologic Options - HT • Should be short-term to treat symptoms in lowest effective dose • Includes… ▫ Estrogens and progestins �Variety of doses �Cyclic or continuous regimens �Variety of routes of administration

Types of Estrogens • • • Conjugated equine estrogens (CEE) Synthetic plant-derived products (Enjuvia) Ethinyl estradiol Micronized estradiol 17 -estradiol (transdermal/implanted/percutaneous) • Esterified estrogens • Dose - start at lowest dose possible ▫ lower dose products – 0. 3 mg, 0. 45 mg

Estrogens • Multiple routes of administration are available • Advantages may extend beyond convenience ▫ Oral ▫ Vaginal �Can be local or systemic effect (depending on the dose used) �Low dose – 0. 3 mg CEE daily (0. 5 g or 1/8 applicator) �Cream, Ring (q 3 mo), Suppository ▫ Transdermal �avoid first-pass metabolism �Patch, Gel, spray

Risk of exposure to estradiol transdermal spray (FDA advisory – 7/10) • children and pets exposure to hormones ▫ ▫ • • 8 case reports between 7/07 – 6/10 Children age 3 -5 yrs were exposed Nipple swelling and breast enlargement Can occur from touching medicated skin 2 reports in dogs, size is a factor No contact with treated skin - 30 min after application Wear garment that covers treated area (after 2 minutes) If contact occurs: ▫ Wash child’s skin with soap and water immediately ▫ Watch for signs of estrogen effects

Progestins • For women with intact uterus • Minimum of 10 -14 days/month • Include: Mostly PO forms ▫ ▫ ▫ ▫ Dydrogesterone Medroxyprogesterone acetate Micronized progesterone Norethindrone acetate Norgestrel Levonorgestrel Drosperinone • ADRs: irritability, depression, HA

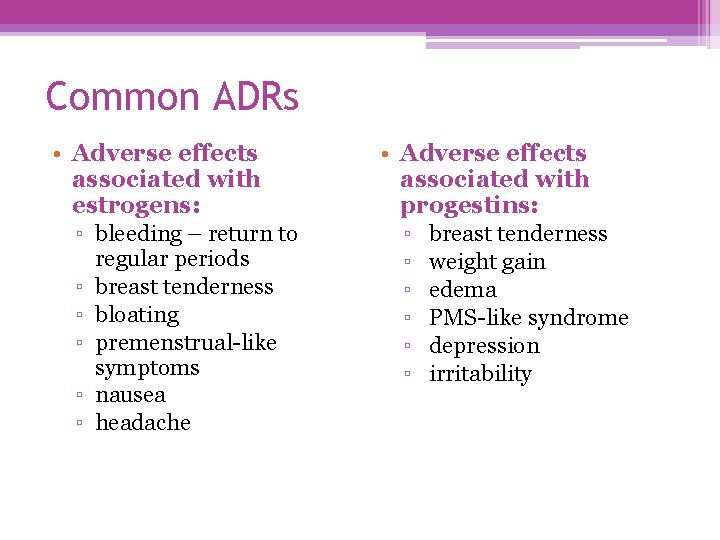
Common ADRs • Adverse effects associated with estrogens: ▫ bleeding – return to ▫ ▫ ▫ regular periods breast tenderness bloating premenstrual-like symptoms nausea headache • Adverse effects associated with progestins: ▫ breast tenderness ▫ weight gain ▫ edema ▫ PMS-like syndrome ▫ depression ▫ irritability

Serious ADRs from HT • Increased risk of: ▫ endometrial cancer �after a period of amenorrhea, heavier than normal or at unscheduled time, refer patient to be checked for possible endometrial cancer ▫ ▫ VTE gallbladder disease breast cancer? CVD and dementia?

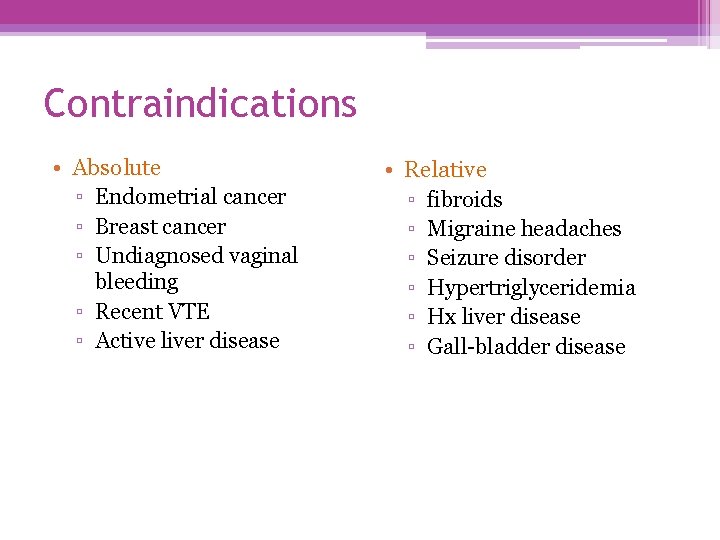
Contraindications • Absolute ▫ Endometrial cancer ▫ Breast cancer ▫ Undiagnosed vaginal bleeding ▫ Recent VTE ▫ Active liver disease • Relative ▫ fibroids ▫ Migraine headaches ▫ Seizure disorder ▫ Hypertriglyceridemia ▫ Hx liver disease ▫ Gall-bladder disease

Cyclic vs. Continuous • Cyclic ▫ Estrogen + progestin for 12 days minimum ▫ Newly diagnosed or bleeding problems • Continuous ▫ Estrogen + progestin every day; better compliance ▫ Irregular bleeding for 6 -12 months • Long Cycle ▫ Progestin added to estrogen every other month for 12 -14 days ▫ Decreases number of periods but period may be heavier • Intermittent ▫ Pulsed therapy of 3 days estrogen followed by 3 days of estrogen + progestin ▫ Less progestin side effects

Androgens • Deficiency may occur in menopause • Symptoms include: ▫ Fatigue, ↓ sense of well-being, sexual function changes • Common regimens include: ▫ Esterified estrogen + 1. 25 or 2. 5 mg methyltestosterone • Long term safety and efficacy studies are lacking

SERMS • Nonsteroidal cpds - bind to estrogen receptors • Combination of agonist/antagonist actions • Ideal: ▫ Protect against osteoporosis ▫ No effect on breast, endometrium ▫ No menopausal-like ADRs ▫ No SERM currently meets these goals • Raloxifene (Evista) • s bone loss, no effect on endometrium, estrogenic action on lipids, risk of VTE similar to CEE • ADRs - hot flashes, migraines, leg cramps, wt gain

Treatment of Postmenopausal Dyspareunia • • • Ospemifene (Osphena) approved 2/26/13 Dyspareunia caused by vulvovaginal atrophy Novel SERM Taken once daily with food Boxed warning for endometrial stimulation Other ADRs – DVT, stroke ▫ Lower risk than estrogen alone ▫ Hot flashes also possible

Duavee • Combination of CEE and bazedoxifene, a SERM • For treatment of vasomotor symptoms in women with a uterus • The SERM substitutes for progestin in the HT therapy regimen • Can also be used to prevent osteoporosis in women with significant risk factors

Non-hormonal therapies for vasomotor symptoms • SNRIs ▫ Venlafaxine 75 mg po qd significantly ↓ compared to placebo • SSRIs ▫ paroxetine has been shown in randomized trials to ↓ hot flashes significantly ▫ Paroxetine (Brisdelle) approved in 2013 for moderate to severe hot flashes (7. 5 mg at bedtime) • Gabapentin ▫ High dose (900 mg) ↓ hot flashes slightly • Clonidine ▫ 0. 1 -. 2 mg produced variable results; bothersome ADRs • High dose MPA ▫ 20 mg/day beneficial ; ADRs not tolerated well by patients

Alternatives to ET/HT • Use of HT plummeted after WHI study • Patients and physicians pursued alternatives ▫ Herbs ▫ Supplements ▫ BHRT • Regimens promoted by celebrities Ageless: The Naked Truth About Bioidentical Hormones

Bioidentical Hormone Therapy (BHT) • "bioidentical" = contains hormones identical to naturally produced hormones ▫ Manufactured products containing estradiol and progesterone ▫ custom compounded recipes identical to human hormones • Natural products used to make BHRT include soy and yam • Compounds are tailored to women’s individual hormone needs ▫ individualized doses , dosage forms and mixtures not available commercially

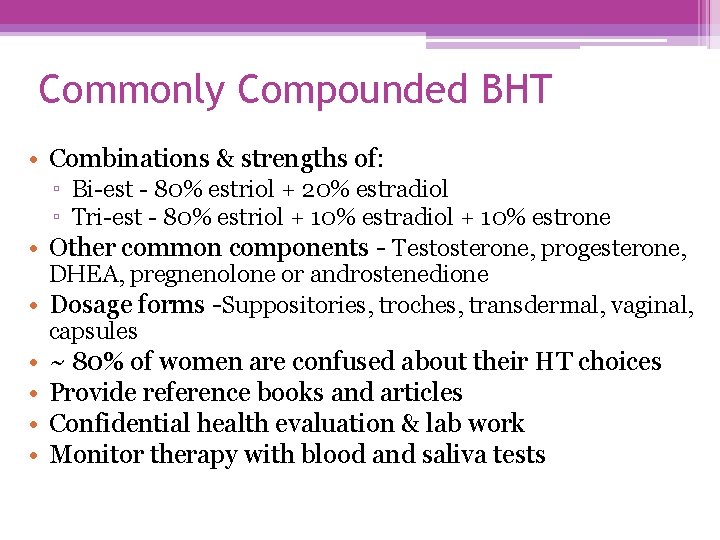
Commonly Compounded BHT • Combinations & strengths of: ▫ Bi-est - 80% estriol + 20% estradiol ▫ Tri-est - 80% estriol + 10% estradiol + 10% estrone • Other common components - Testosterone, progesterone, DHEA, pregnenolone or androstenedione • Dosage forms -Suppositories, troches, transdermal, vaginal, capsules • • ~ 80% of women are confused about their HT choices Provide reference books and articles Confidential health evaluation & lab work Monitor therapy with blood and saliva tests

Is BHT Safer or More Efficacious than MHT? The case against: • MHT is approved by FDA as safe and efficacious following large clinical trials • Manufacturers are federally regulated to ensure the purity, quality and safety of their products ▫ Quality control over purity and dosing accuracy is responsibility of the compounder • Wealth of clinical studies and publications on MHT ▫ Current lack of clinical data for BHT -North American Menopause Society -National Institute on Aging

Is BHT Safer or More Efficacious than MHT? The case for: • “Natural” products are preferred over synthetic products • Chemically identical to hormones in the body • Custom made with a broad selection of doses and combinations • “High touch” therapy Professional Compounding Centers of America

The Bottom Line: ▫ We will not be able to adequately compare the risks and benefits of MHT vs. BHT until appropriate scientific evidence is gathered ▫ In the meantime, all of the available treatments and evidence regarding these treatments should be made known and available to each patient

Natural products for menopausal symptoms • Alternatives to HT • $600 million spent on natural products (2000) • After WHI report, natural product popularity exploded • Most commonly used ▫ Phytoestrogen ▫ Herbs ▫ Bioidentical hormones

Phytoestrogens • • Plant estrogens - isoflavones, lignans, coumestans Commonly found in food Bind to estrogen receptors 100 -10, 000 times weaker than estrogen ▫ Can act like SERMS or estrogen • Can they prevent hot flashes? ▫ Only 10% of Asian women have hot flashes • Dose normally given – 20 -60 gm of soy, 34 -76 mg isoflavone – some studies show modest improvement

Are phytoestrogen ADRS similar to estrogen? • Common ADRs ▫ Constipation, bloating, nausea • Breast cancer ▫ There is conflicting evidence • Endometrial cancer ▫ No evidence to suggest risk without progestin • ↓LDL, cholesterol, TGs; no effect on HDLs • Safest recommendation – use food sources not high dose supplement products

Other herbs promoted for menopause • Red Clover ▫ Contains isoflavones and coumarins • Flaxseed ▫ Lignans, omega-3 FAs, fiber; contains fat and calories • Black Cohosh ▫ Top-selling product (Remifemin) ▫ Study demonstrated effectiveness equal to low dose patch for hot flashes ▫ Don’t confuse with blue or white cohosh • Dong quai - Not shown effective; may contain carcinogens

Other herbs promoted for menopause • Chasteberry ▫ Affects dopamine and acetylcholine; may help PMS symptoms • Kudzu, alfalfa, hops and licorice have estrogenic effects but are not shown to be effective for menopause • DHEA ▫ Proposed anti-aging hormone ▫ May raise testosterone levels and cause androgenic ADRs ▫ May increase risk of breast cancer

Other herbs promoted for menopause • Evening primrose oil and vitamin E ▫ Not shown to be effective • Wild yam – can be converted to hormones in the lab, not in the body • Take home message: ▫ Herbs and supplements may NOT be safer ▫ Soy or other phytoestrogens may help moderately �Food sources are preferred ▫ Others are not proven effective and some may be toxic

Summarize recommendation for HT • For moderate to severe vasomotor symptoms ▫ Use lowest effective systemic dose • For vaginal symptoms only ▫ Use local estrogen or lubricants • Decision to use HT is patient choice in conjunction with health professional ▫ For patients who can’t or choose not to use hormones, look at alternatives • Still many unanswered questions ▫ Are bioidentical, certain forms of estrogen and progestin, or transdermals better?

What do you need to know to assist a patient in her decision? • • Medical history Family history Social history Physical exam Test results Laboratory results Be able to share the pros and cons of using HT

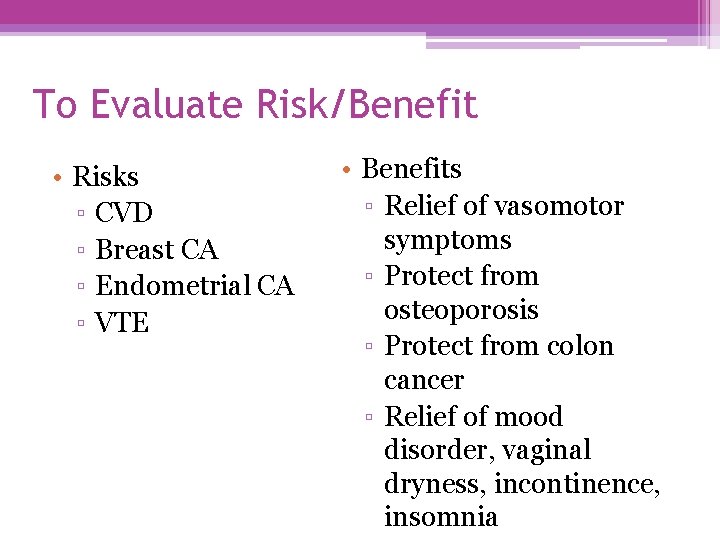
To Evaluate Risk/Benefit • Risks ▫ CVD ▫ Breast CA ▫ Endometrial CA ▫ VTE • Benefits ▫ Relief of vasomotor symptoms ▫ Protect from osteoporosis ▫ Protect from colon cancer ▫ Relief of mood disorder, vaginal dryness, incontinence, insomnia


Case 1 RM, a 46 yo female complains of heavy prolonged menstrual periods and difficulty sleeping due to night sweats. • What questions would you ask to gather information to assist the patient in choosing a course of action? • What is the most commonly used agent to treat her symptoms?

Case 2 DP, age 54, describes herself as having gone through “the change. ” She is thinking she needs to start on hormone replacement. What do you think?

Case 3 ZK, a 55 yo teacher, experiences drenching sweats at night and has difficulty sleeping. She has trouble concentrating during the day. She is concerned about HT because she read it might be dangerous. She is thinking about something “natural. ”
 Marquette nfp perimenopause
Marquette nfp perimenopause Cmhrs services
Cmhrs services Ppd-946
Ppd-946 Stat 946
Stat 946 Dr wilson adrenal rebuilder side effects
Dr wilson adrenal rebuilder side effects Symplex f for menopause
Symplex f for menopause Menopause definition
Menopause definition When is menopause
When is menopause Premature menopause quiz
Premature menopause quiz Menopause wann
Menopause wann Minal jadhav
Minal jadhav Pps emergency signals and actions
Pps emergency signals and actions Pps scale
Pps scale Pps marrant
Pps marrant Pps score
Pps score Pps chansons
Pps chansons Pps lončki
Pps lončki Cidb pps requirement
Cidb pps requirement Opis rzeźby kaliope
Opis rzeźby kaliope Pps mania
Pps mania Post dam area in complete denture
Post dam area in complete denture Tina medved
Tina medved Pps survey
Pps survey Pps southern tagalog chapter
Pps southern tagalog chapter Pps diaporamas amusants
Pps diaporamas amusants Loulous pps biz
Loulous pps biz Pps loulou
Pps loulou Humour pps
Humour pps Loulou pps
Loulou pps Diaporamas gratuits
Diaporamas gratuits Diaporamas gratuits
Diaporamas gratuits Kamdou net
Kamdou net Lilymage pps
Lilymage pps Ppssexy
Ppssexy Sexy pps
Sexy pps Ranks vs phrases
Ranks vs phrases Pps summer school
Pps summer school Pps josefina
Pps josefina Pps josefina
Pps josefina Diseado
Diseado Pps a la con
Pps a la con Mein leben. pps
Mein leben. pps Pps scale hospice
Pps scale hospice Pps scale hospice
Pps scale hospice Pps magda
Pps magda Pps brief
Pps brief P.p.s. brief
P.p.s. brief Advent pps
Advent pps Pps sexy
Pps sexy Pps diaporamas amusants
Pps diaporamas amusants Diaporamas humoristiques
Diaporamas humoristiques