Treatment of Parkinsons Disease and Movement Disorders David









- Slides: 24

Treatment of Parkinson’s Disease and Movement Disorders David G. Standaert, M. D. , Ph. D. Massachusetts General Hospital. Harvard Medical School

Movement Disorders n n n Parkinson’s Disease Tremor Chorea Ballism Dystonia Tic Disorders

Parkinson’s disease n n Described by. James Parkinson, 1817 Most common disorder of movement Affects 3% of the population overthe age of 65 years About 500, 000 patients in the US

“Cardinal Features” of Parkinson’s Disease n n Tremor Rigidity Bradykinesia Postural Instability

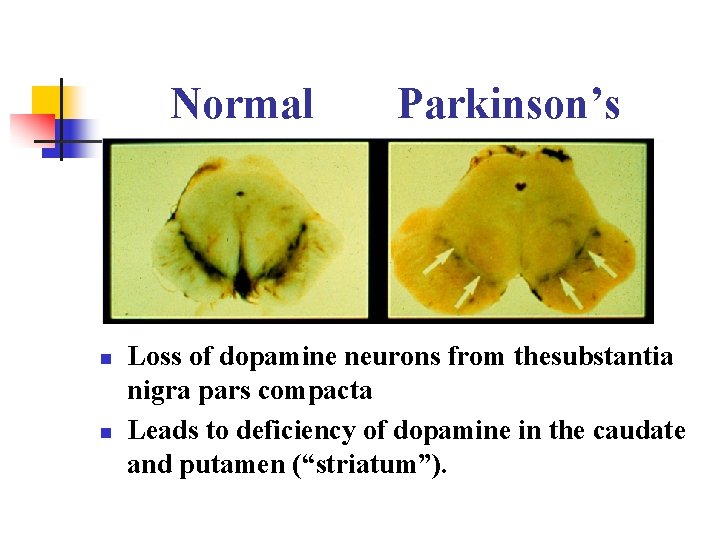
Normal n n Parkinson’s Loss of dopamine neurons from thesubstantia nigra pars compacta Leads to deficiency of dopamine in the caudate and putamen (“striatum”).


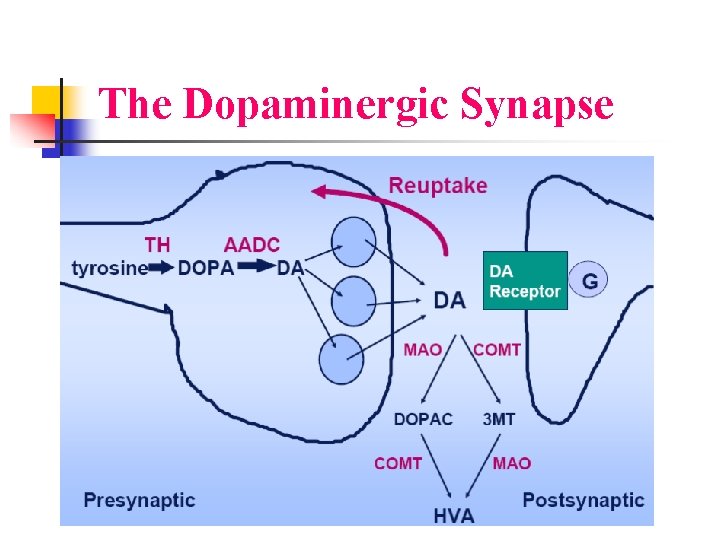
The Dopaminergic Synapse

Dopamine Receptors n Classical Pharmacology: n n n D 1 -stimulates c. AMP formation D 2 -inhibits c. AMP formation Molecular Pharmacology: n n Family of at least 5 receptor proteins All have 7 transmembrane regions, typical of. Gprotein coupled receptors d 1 and d 2 are abundant in striatum, correspond to classically identified sites Others primarily extrastriatal, likely accountfor many of the side effects of dopaminergicdrugs

Pharmacological Approaches to. Treatment of Parkinson’s Disease n Symptomatic treatments n n most are based on dopamineaugmentation “Neuroprotective” treatments n n none presently proven most current studies are based on “oxidative stress hypothesis”

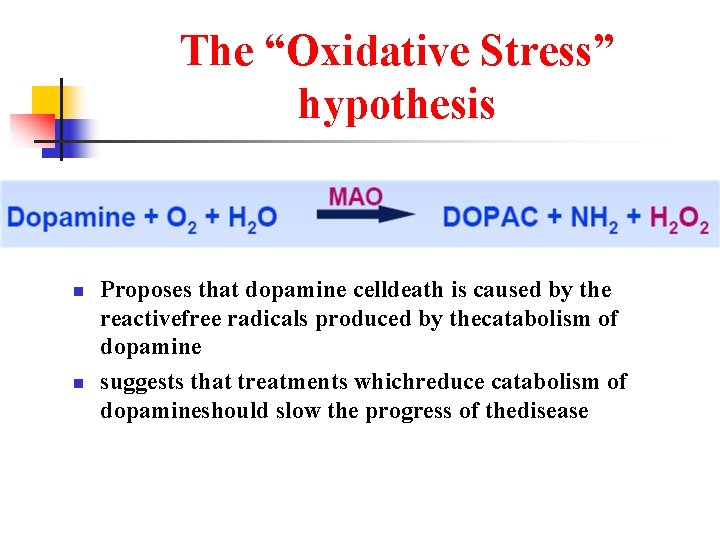
The “Oxidative Stress” hypothesis n n Proposes that dopamine celldeath is caused by the reactivefree radicals produced by thecatabolism of dopamine suggests that treatments whichreduce catabolism of dopamineshould slow the progress of thedisease

Levodopa therapy n n n also called L-DOPA, L-dihydroxyphenylalanine Works by replacing biosynthetic precursor: Usually given with carbidopa, an inhibitor of peripheral AADC -prevents nausea. Adverse effects: peripheral, central Most important limitation of treatment is the development of “complications of levodopa therapy” wearing off and dyskinesias

Levodopa Therapy of Parkinson’s Disease n n n 1950’s: Arvid Carlsson discovers that dopamine is a neurotransmitter, reserpine replicates features of. Parkinson’s 1960: Deficiency of dopamine inpostmortem PD described by Enringer and Hornykeiwicz 1961: Effect of levodopa in PD reportedby Birkmayer and Hornykeiwicz 1967: Long term treatment of PD withlevodopa described by Cotzias et al. 2000: Carlsson, Kandel and Greengardawarded Nobel prize

Motor complicationsof levodopa therapy n n n Fluctuations: variations in mobility related to medication dose and interval. Wearing-off: loss of efficacy at the end of a dosing interval Dyskinesias: excessive, involuntary movements

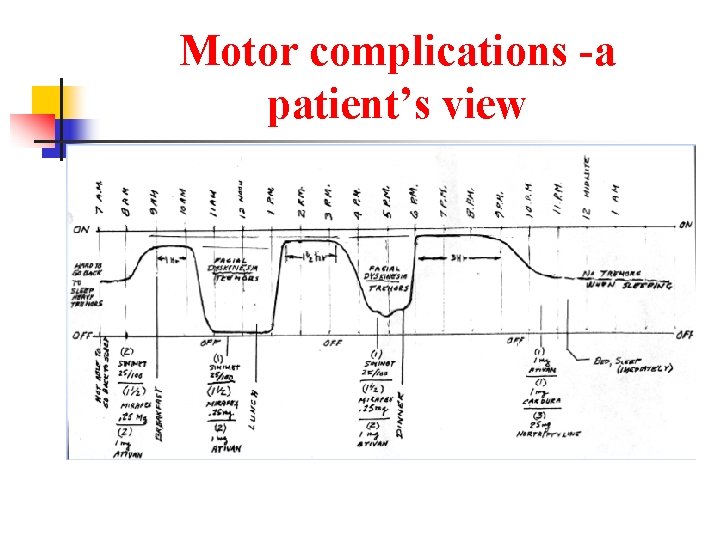
Motor complications -a patient’s view

What causes fluctuations, wearing off, and dyskinesias? n n Not explained by simple DA receptorupregulation Loss of “buffering capacity” is animportant factor Clinical and experimental data suggeststhat variations in plasma levodopalevels have an important “inductive”effect Role of NMDA glutamate receptors

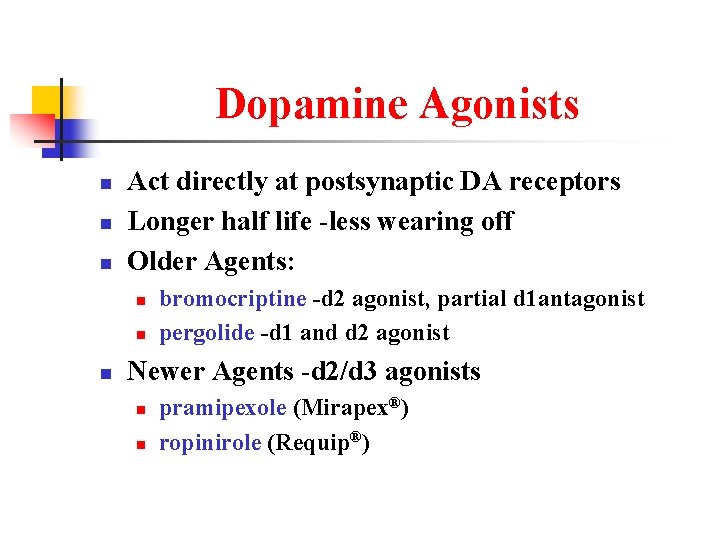
Dopamine Agonists n n n Act directly at postsynaptic DA receptors Longer half life -less wearing off Older Agents: n n n bromocriptine -d 2 agonist, partial d 1 antagonist pergolide -d 1 and d 2 agonist Newer Agents -d 2/d 3 agonists n n pramipexole (Mirapex®) ropinirole (Requip®)

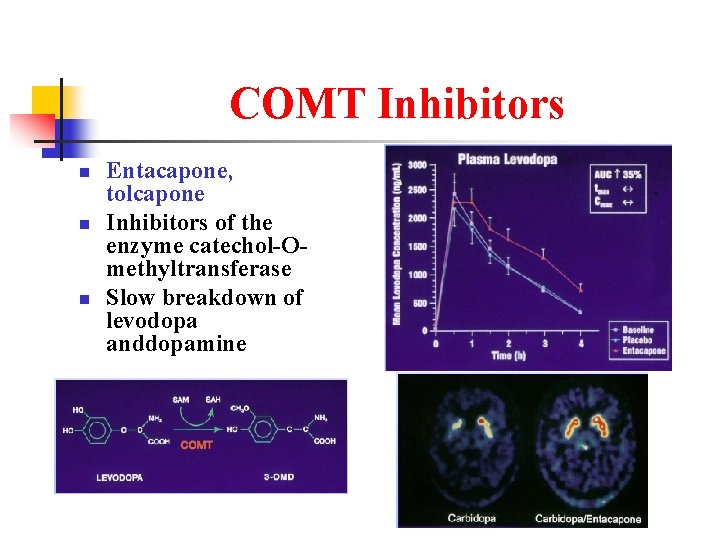
COMT Inhibitors n n n Entacapone, tolcapone Inhibitors of the enzyme catechol-Omethyltransferase Slow breakdown of levodopa anddopamine

Motor complications of levodopa: prevention? n n Hypothesis: “nonphysiologic”replacement of dopamine by orallevodopa underlies the developmentof motor complications Dopamine agonists: a “morephysiologic” replacement

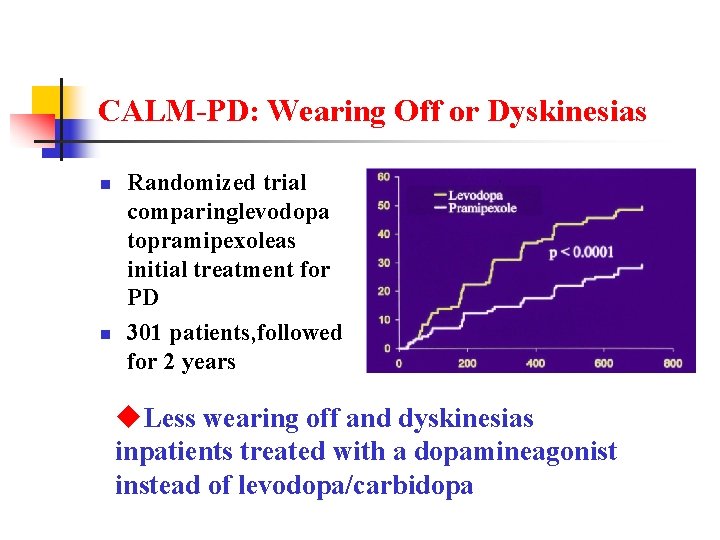
CALM-PD: Wearing Off or Dyskinesias n n Randomized trial comparinglevodopa topramipexoleas initial treatment for PD 301 patients, followed for 2 years u. Less wearing off and dyskinesias inpatients treated with a dopamineagonist instead of levodopa/carbidopa

Dopamine agonists as initial therapy n n Initial treatment with pramipexole or ropinirole instead of levodopa reduces development of wearing off or dyskinesias. But this comes at a price: n n Increased fatigue and somnolence Increased hallucinations in the elderly ? Reduced efficacy Increased cost

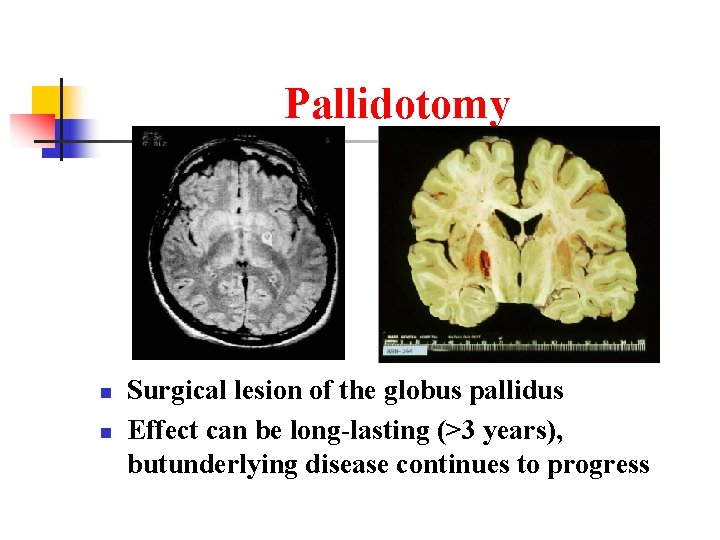
Pallidotomy n n Surgical lesion of the globus pallidus Effect can be long-lasting (>3 years), butunderlying disease continues to progress

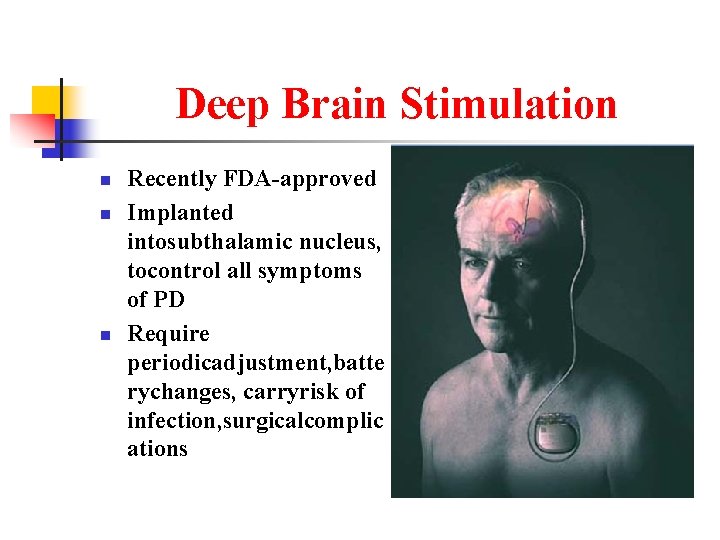
Deep Brain Stimulation n Recently FDA-approved Implanted intosubthalamic nucleus, tocontrol all symptoms of PD Require periodicadjustment, batte rychanges, carryrisk of infection, surgicalcomplic ations

Dopamine receptor antagonists n n Principal application is treatment of psychosis Also used as antiemetics “Typical” antipsychotics n Distinguished by potency at D 2 receptors anddegree of sedation n May cause movement disorders – n Akathisia n Dystonia n Tardive Dyskinesia n “Neuroleptic Malignant Syndrome” “Atypical” antipsychotics n clozapine -d 4 antagonist. Effective in refractorypsychosis, but causes seizures, neutropenia n Resperidone, olanzapine, quetiapine

Dopamine receptor antagonists n n Principal application is treatment of psychosis Also used as antiemetics “Typical” antipsychotics n Distinguished by potency at D 2 receptors anddegree of sedation n May cause movement disorders – n Akathisia n Dystonia n Tardive Dyskinesia n “Neuroleptic Malignant Syndrome” “Atypical” antipsychotics n clozapine -d 4 antagonist. Effective in refractorypsychosis, but causes seizures, neutropenia n Resperidone, olanzapine, quetiapine
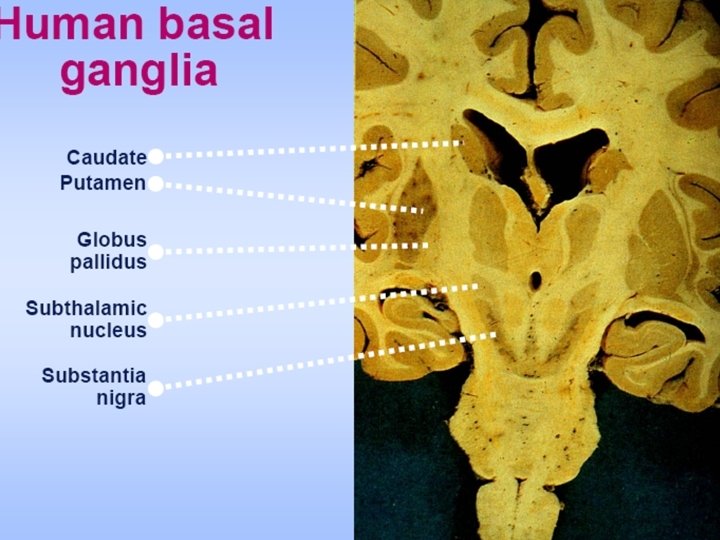
 Basal ganglia
Basal ganglia Tasha big feet
Tasha big feet Shy drager syndrome
Shy drager syndrome Mesorectal fat
Mesorectal fat Katharine hepburn parkinsons
Katharine hepburn parkinsons Parkinsons wiki
Parkinsons wiki Bharathi viswanathan
Bharathi viswanathan Transeuro parkinson's disease
Transeuro parkinson's disease Treatment for prion disease
Treatment for prion disease Nephrotic syndrome
Nephrotic syndrome Maple syrup urine disease treatment
Maple syrup urine disease treatment Modern treatment of heart disease
Modern treatment of heart disease Disease control phase dental
Disease control phase dental Movement and non movement area
Movement and non movement area Locomotor movements dance
Locomotor movements dance Unit 14 health and social care coursework
Unit 14 health and social care coursework Bipolar and other related disorders
Bipolar and other related disorders Bipolar and other related disorders
Bipolar and other related disorders Emotional disturbance assistive technology
Emotional disturbance assistive technology Puberty and autism spectrum disorders
Puberty and autism spectrum disorders Axis 1 and axis 2 disorders
Axis 1 and axis 2 disorders What is neurosis disorder
What is neurosis disorder Define a primary skin lesion and list three types
Define a primary skin lesion and list three types Physical disorders and health psychology
Physical disorders and health psychology Chapter 6 musculoskeletal system diseases and disorders
Chapter 6 musculoskeletal system diseases and disorders














































