Treatment of metastatic pancreatic cancer Can we now



- Slides: 31

Treatment of metastatic pancreatic cancer: Can we now sequence through multiple lines? Andrew H. Ko, MD University of California San Francisco Gastrointestinal Oncology Program 21 st Annual NOCR Meeting, Las Vegas, NV March 13 -14, 2015

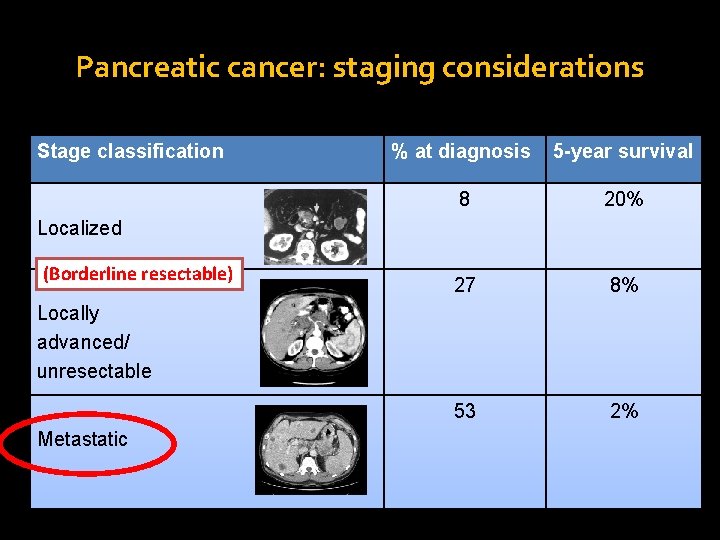
Pancreatic cancer: staging considerations Stage classification % at diagnosis 5 -year survival 8 20% 27 8% 53 2% Localized (Borderline resectable) Locally advanced/ unresectable Metastatic Adapted from Siegel et al, 2012 .

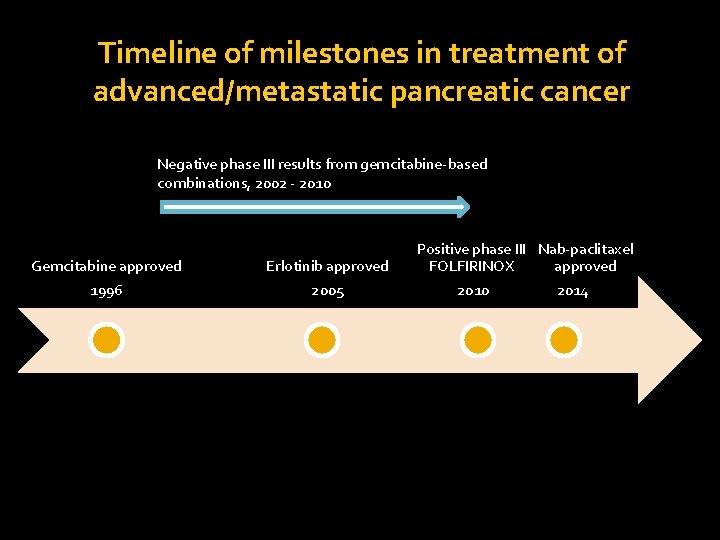
Timeline of milestones in treatment of advanced/metastatic pancreatic cancer Negative phase III results from gemcitabine-based combinations, 2002 - 2010 Gemcitabine approved 1996 Erlotinib approved 2005 Positive phase III Nab-paclitaxel FOLFIRINOX approved 2010 2014

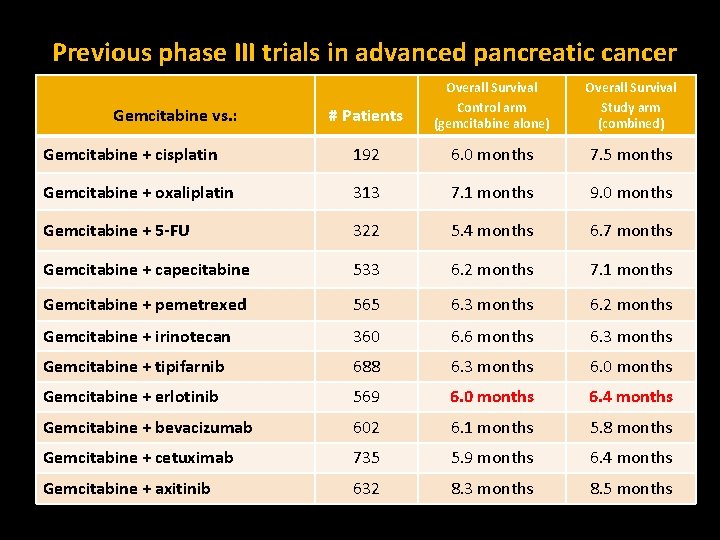
Previous phase III trials in advanced pancreatic cancer # Patients Overall Survival Control arm (gemcitabine alone) Overall Survival Study arm (combined) Gemcitabine + cisplatin 192 6. 0 months 7. 5 months Gemcitabine + oxaliplatin 313 7. 1 months 9. 0 months Gemcitabine + 5 -FU 322 5. 4 months 6. 7 months Gemcitabine + capecitabine 533 6. 2 months 7. 1 months Gemcitabine + pemetrexed 565 6. 3 months 6. 2 months Gemcitabine + irinotecan 360 6. 6 months 6. 3 months Gemcitabine + tipifarnib 688 6. 3 months 6. 0 months Gemcitabine + erlotinib 569 6. 0 months 6. 4 months Gemcitabine + bevacizumab 602 6. 1 months 5. 8 months Gemcitabine + cetuximab 735 5. 9 months 6. 4 months Gemcitabine + axitinib 632 8. 3 months 8. 5 months Gemcitabine vs. :

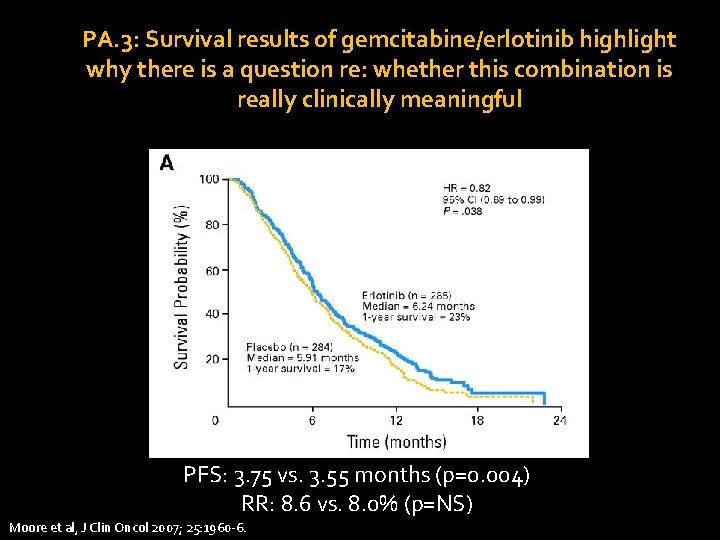
PA. 3: Survival results of gemcitabine/erlotinib highlight why there is a question re: whether this combination is really clinically meaningful PFS: 3. 75 vs. 3. 55 months (p=0. 004) RR: 8. 6 vs. 8. 0% (p=NS) HR = hazard ratio; PFS = progression-free survival; RR = response rate. Moore et al, J Clin Oncol 2007; 25: 1960 -6.

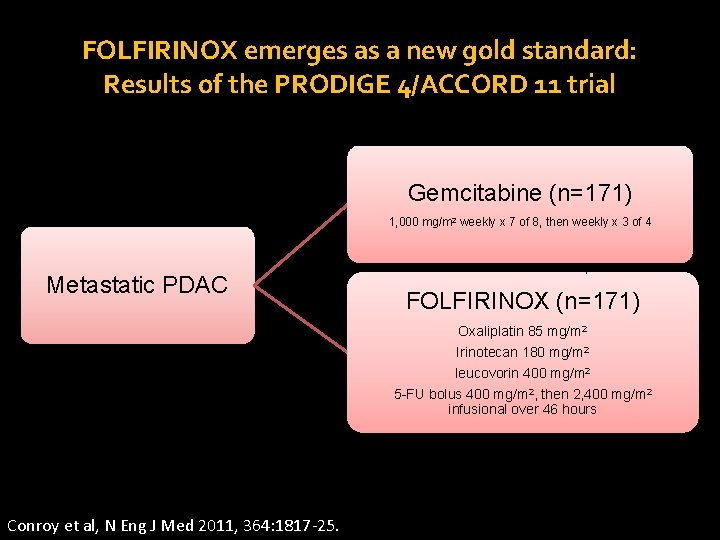
FOLFIRINOX emerges as a new gold standard: Results of the PRODIGE 4/ACCORD 11 trial Gemcitabine (n=171) 1, 000 mg/m 2 weekly x 7 of 8, then weekly x 3 of 4 Metastatic PDAC Stratified by ECOG PS (0 vs. 1), center, tumor location (head vs. other) FOLFIRINOX (n=171) Oxaliplatin 85 mg/m 2 Irinotecan 180 mg/m 2 leucovorin 400 mg/m 2 5 -FU bolus 400 mg/m 2, then 2, 400 mg/m 2 infusional over 46 hours 6 months of chemo planned for each arm Conroy et al, N Eng J Med 2011, 364: 1817 -25.

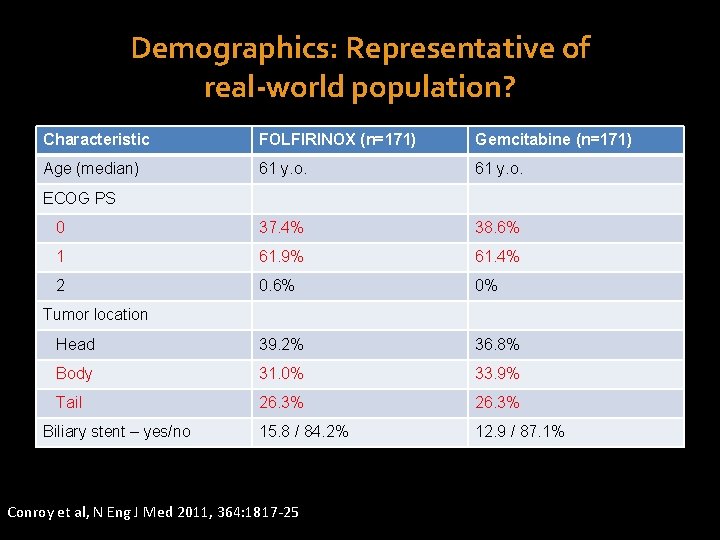
Demographics: Representative of real-world population? Characteristic FOLFIRINOX (n=171) Gemcitabine (n=171) Age (median) 61 y. o. 0 37. 4% 38. 6% 1 61. 9% 61. 4% 2 0. 6% 0% Head 39. 2% 36. 8% Body 31. 0% 33. 9% Tail 26. 3% 15. 8 / 84. 2% 12. 9 / 87. 1% ECOG PS Tumor location Biliary stent – yes/no Conroy et al, N Eng J Med 2011, 364: 1817 -25

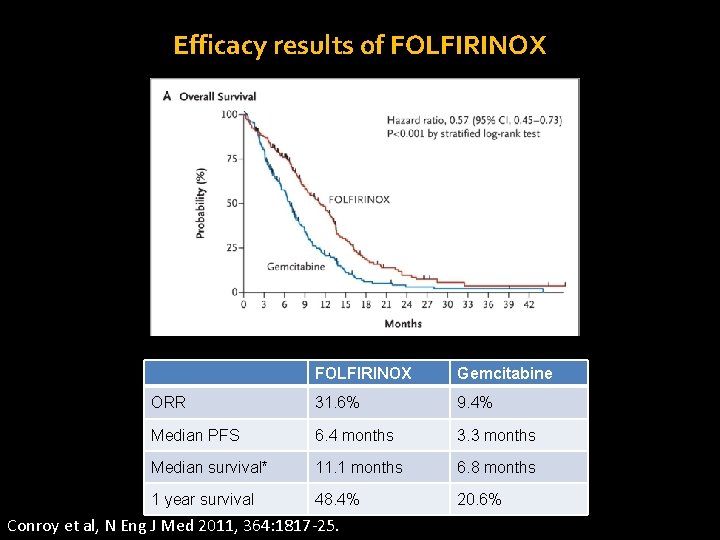
Efficacy results of FOLFIRINOX Gemcitabine ORR 31. 6% 9. 4% Median PFS 6. 4 months 3. 3 months Median survival* 11. 1 months 6. 8 months 1 year survival 48. 4% 20. 6% Conroy et al, N Eng J Med 2011, 364: 1817 -25.

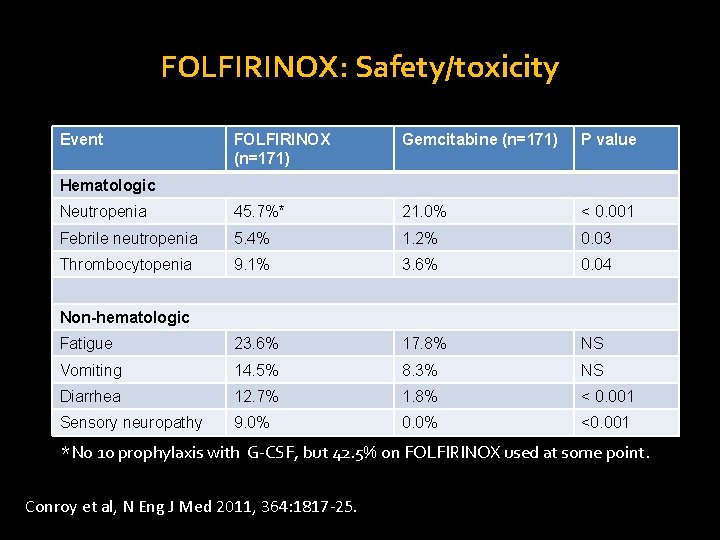
FOLFIRINOX: Safety/toxicity Event FOLFIRINOX (n=171) Gemcitabine (n=171) P value Neutropenia 45. 7%* 21. 0% < 0. 001 Febrile neutropenia 5. 4% 1. 2% 0. 03 Thrombocytopenia 9. 1% 3. 6% 0. 04 Fatigue 23. 6% 17. 8% NS Vomiting 14. 5% 8. 3% NS Diarrhea 12. 7% 1. 8% < 0. 001 Hematologic Non-hematologic Sensory neuropathy 9. 0% 0. 0% <0. 001 *No 1 o prophylaxis with G-CSF, but 42. 5% on FOLFIRINOX used at some point. Conroy et al, N Eng J Med 2011, 364: 1817 -25.

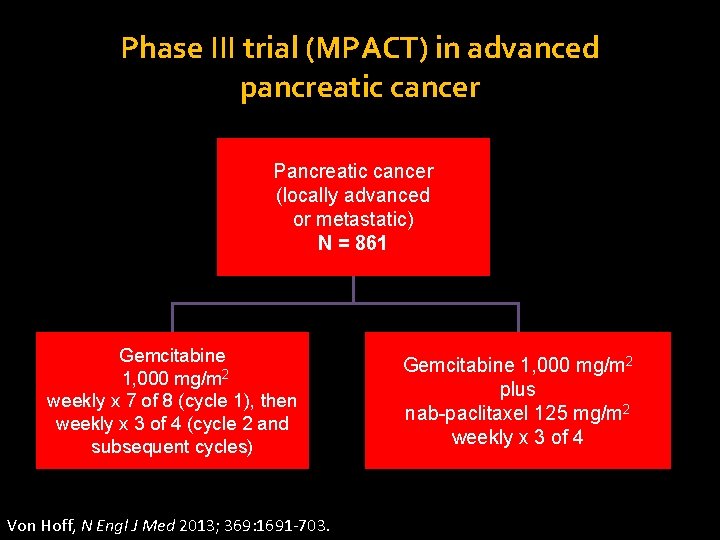
Phase III trial (MPACT) in advanced pancreatic cancer Pancreatic cancer (locally advanced or metastatic) N = 861 Gemcitabine 1, 000 mg/m 2 weekly x 7 of 8 (cycle 1), then weekly x 3 of 4 (cycle 2 and subsequent cycles) Von Hoff, N Engl J Med 2013; 369: 1691 -703. Stratification by KPS, region, liver metastases Gemcitabine 1, 000 mg/m 2 plus nab-paclitaxel 125 mg/m 2 weekly x 3 of 4

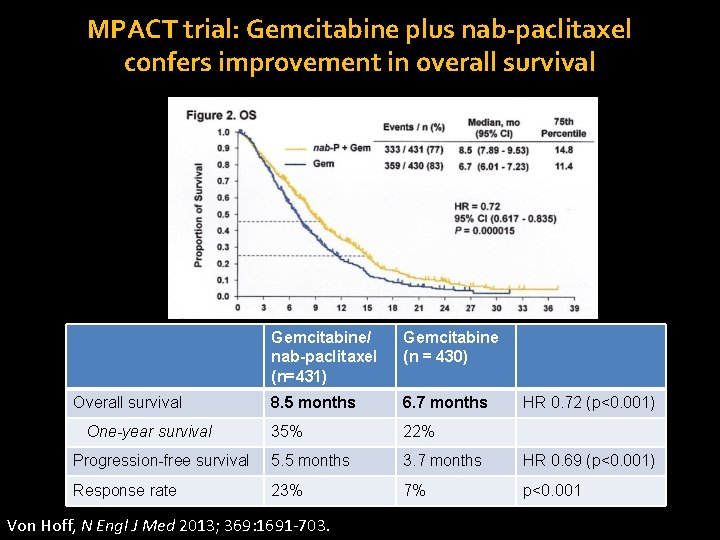
MPACT trial: Gemcitabine plus nab-paclitaxel confers improvement in overall survival Gemcitabine/ nab-paclitaxel (n=431) Gemcitabine (n = 430) 8. 5 months 6. 7 months 35% 22% Progression-free survival 5. 5 months 3. 7 months HR 0. 69 (p<0. 001) Response rate 23% 7% p<0. 001 Overall survival One-year survival Von Hoff, N Engl J Med 2013; 369: 1691 -703. HR 0. 72 (p<0. 001)

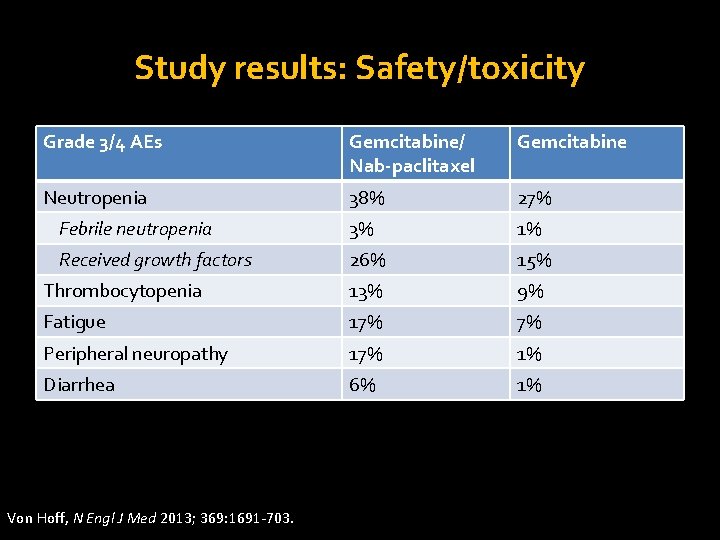
Study results: Safety/toxicity Grade 3/4 AEs Gemcitabine/ Nab-paclitaxel Gemcitabine Neutropenia 38% 27% Febrile neutropenia 3% 1% Received growth factors 26% 15% Thrombocytopenia 13% 9% Fatigue 17% 7% Peripheral neuropathy 17% 1% Diarrhea 6% 1% Von Hoff, N Engl J Med 2013; 369: 1691 -703.

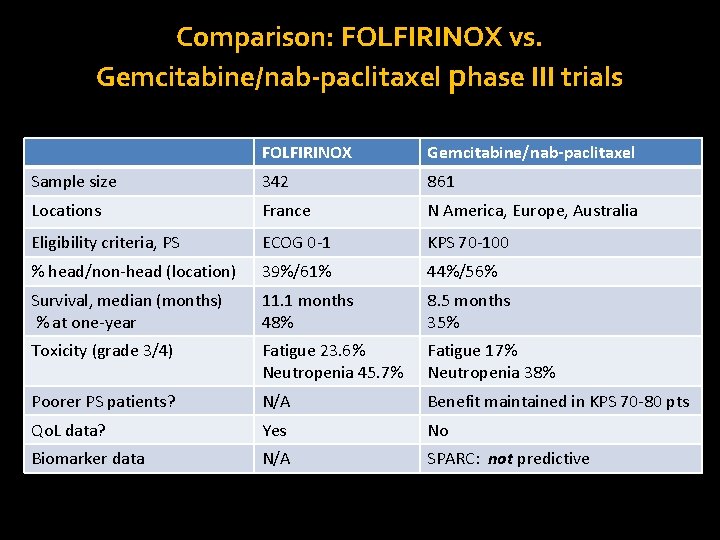
Comparison: FOLFIRINOX vs. Gemcitabine/nab-paclitaxel phase III trials FOLFIRINOX Gemcitabine/nab-paclitaxel Sample size 342 861 Locations France N America, Europe, Australia Eligibility criteria, PS ECOG 0 -1 KPS 70 -100 % head/non-head (location) 39%/61% 44%/56% Survival, median (months) % at one-year 11. 1 months 48% 8. 5 months 35% Toxicity (grade 3/4) Fatigue 23. 6% Neutropenia 45. 7% Fatigue 17% Neutropenia 38% Poorer PS patients? N/A Benefit maintained in KPS 70 -80 pts Qo. L data? Yes No Biomarker data N/A SPARC: not predictive

Beyond front-line therapy for metastatic PDAC • Historically, ~ 50% of patients are suitable candidates for post-progression treatment (Schrag, J Clin Oncol 2007 [abstract]) – Many with rapid clinical/functional decline and inanition – There has previously been an absence of other good therapeutic options! – Traditionally, a patient’s first shot was his/her best (and oftentimes only) shot • With newer and somewhat more effective treatment options, are we changing the biology and course of patients’ disease sufficiently such that there is a more pressing need to think about 2 nd- (and 3 rd, and 4 th…) line therapies?

Key point: o Although we have an expanding array of therapeutic options for advanced pancreatic cancer, currently there is NO established standard of care for patients who have progressed on first-line treatment; moreover, we have NO validated biomarkers that help guide us in therapeutic decision-making.

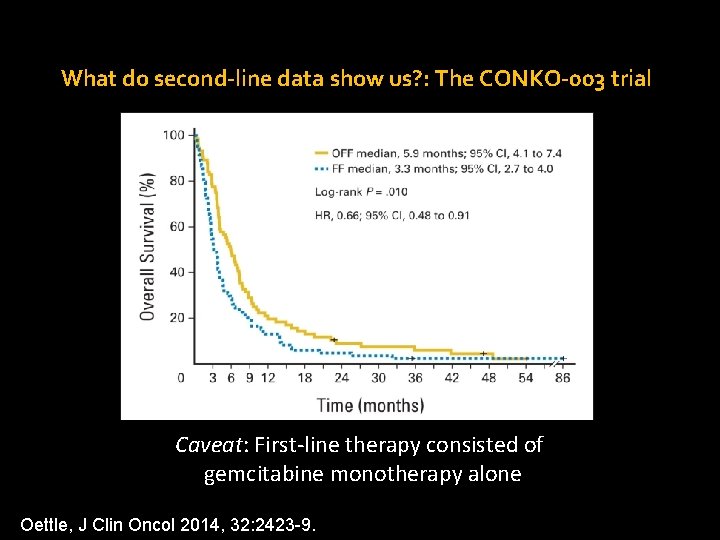
What do second-line data show us? : The CONKO-003 trial Caveat: First-line therapy consisted of gemcitabine monotherapy alone Oettle, J Clin Oncol 2014, 32: 2423 -9.

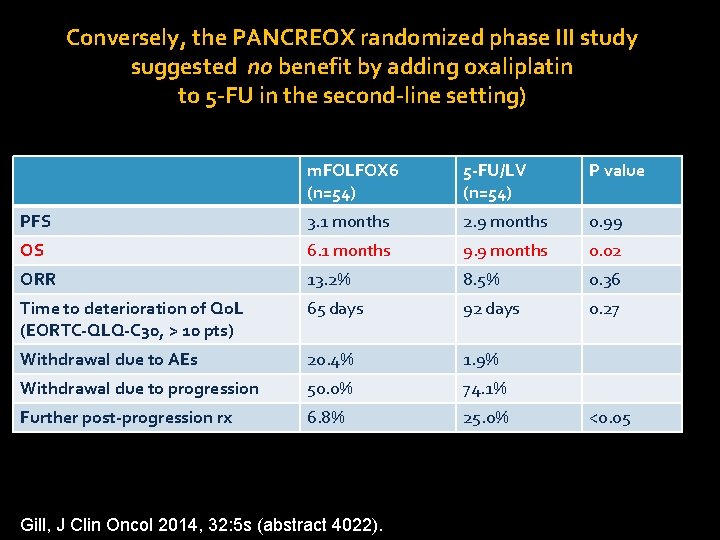
Conversely, the PANCREOX randomized phase III study suggested no benefit by adding oxaliplatin to 5 -FU in the second-line setting) m. FOLFOX 6 (n=54) 5 -FU/LV (n=54) P value PFS 3. 1 months 2. 9 months 0. 99 OS 6. 1 months 9. 9 months 0. 02 ORR 13. 2% 8. 5% 0. 36 Time to deterioration of Qo. L (EORTC-QLQ-C 30, > 10 pts) 65 days 92 days 0. 27 Withdrawal due to AEs 20. 4% 1. 9% Withdrawal due to progression 50. 0% 74. 1% Further post-progression rx 6. 8% 25. 0% Gill, J Clin Oncol 2014, 32: 5 s (abstract 4022). <0. 05

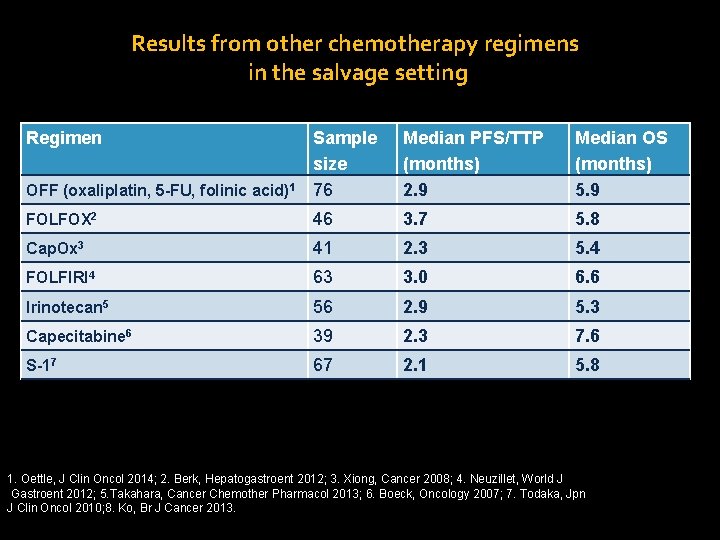
Results from other chemotherapy regimens in the salvage setting Regimen OFF (oxaliplatin, 5 -FU, folinic acid)1 Sample size 76 Median PFS/TTP (months) 2. 9 Median OS (months) 5. 9 FOLFOX 2 46 3. 7 5. 8 Cap. Ox 3 41 2. 3 5. 4 FOLFIRI 4 63 3. 0 6. 6 Irinotecan 5 56 2. 9 5. 3 Capecitabine 6 39 2. 3 7. 6 S-17 67 2. 1 5. 8 1. Oettle, J Clin Oncol 2014; 2. Berk, Hepatogastroent 2012; 3. Xiong, Cancer 2008; 4. Neuzillet, World J Gastroent 2012; 5. Takahara, Cancer Chemother Pharmacol 2013; 6. Boeck, Oncology 2007; 7. Todaka, Jpn J Clin Oncol 2010; 8. Ko, Br J Cancer 2013.

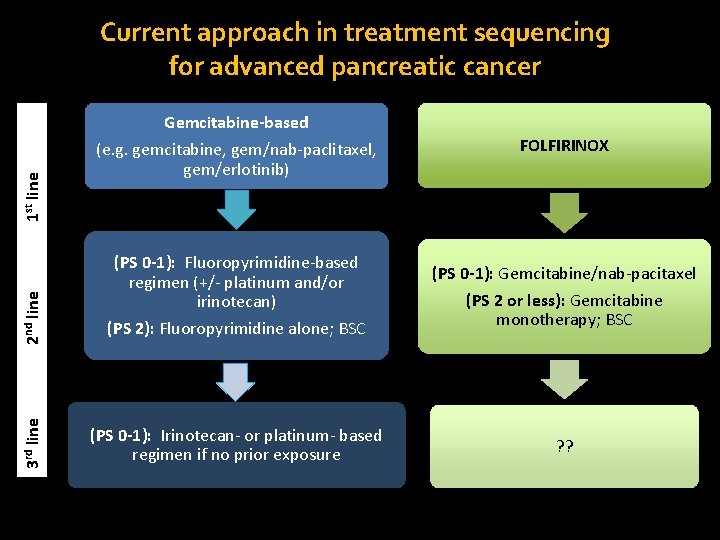
FOLFIRINOX 2 nd line (PS 0 -1): Fluoropyrimidine-based regimen (+/- platinum and/or irinotecan) (PS 2): Fluoropyrimidine alone; BSC (PS 0 -1): Gemcitabine/nab-pacitaxel (PS 2 or less): Gemcitabine monotherapy; BSC (PS 0 -1): Irinotecan- or platinum- based regimen if no prior exposure ? ? 1 st line Gemcitabine-based (e. g. gemcitabine, gem/nab-paclitaxel, gem/erlotinib) 3 rd line Current approach in treatment sequencing for advanced pancreatic cancer

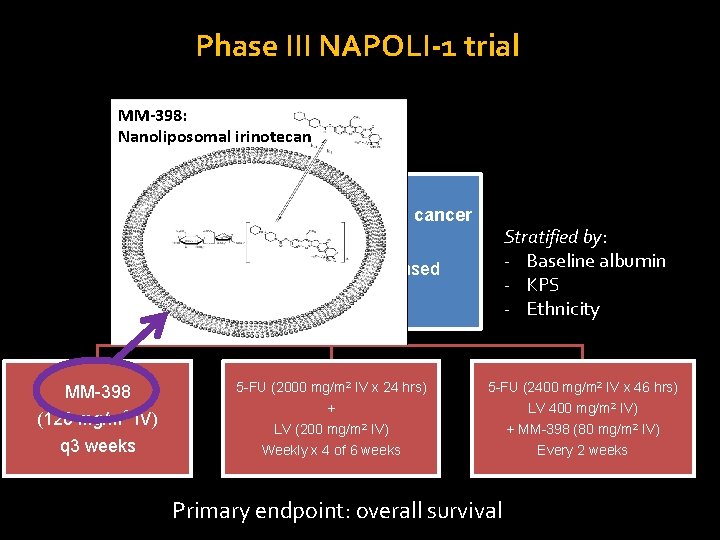
Phase III NAPOLI-1 trial MM-398: Nanoliposomal irinotecan Metastatic pancreatic cancer Stratified by: - Baseline albumin - KPS - Ethnicity N = 417 s/p gemcitabine-based chemotherapy MM-398 mg/m 2 (120 IV) q 3 weeks 5 -FU (2000 mg/m 2 IV x 24 hrs) 5 -FU (2400 mg/m 2 IV x 46 hrs) + LV 400 mg/m 2 IV) + MM-398 (80 mg/m 2 IV) Every 2 weeks LV (200 mg/m 2 IV) Weekly x 4 of 6 weeks Primary endpoint: overall survival

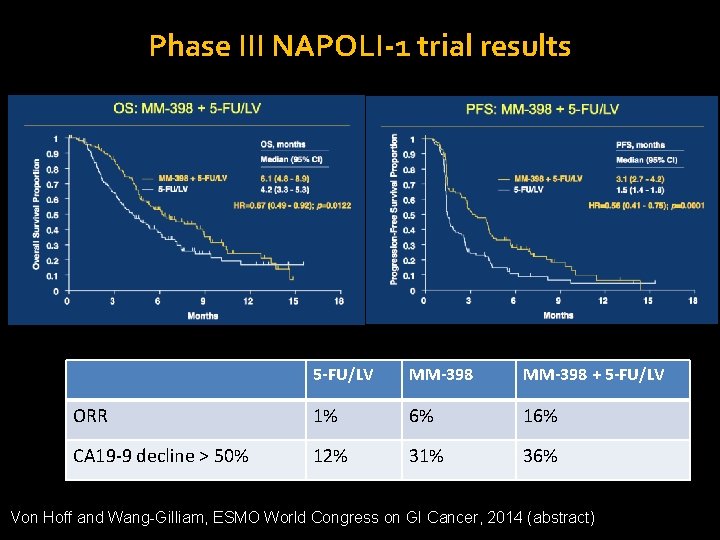
Phase III NAPOLI-1 trial results 5 -FU/LV MM-398 + 5 -FU/LV ORR 1% 6% 16% CA 19 -9 decline > 50% 12% 31% 36% Von Hoff and Wang-Gilliam, ESMO World Congress on GI Cancer, 2014 (abstract)

Where will MM-398 fit into the treatment paradigm for advanced PDAC? • Currently under review for FDA approval • Probably only following a front-line gemcitabinebased regimen; would not expect much activity post. FOLFIRINOX (overlap of essentially same drug components) • How much better is MM-398/5 -FU/LV compared to, say, FOLFIRI? • Potential treatment sequencing as second- or thirdline therapy (either before or after platinum-based regimen)

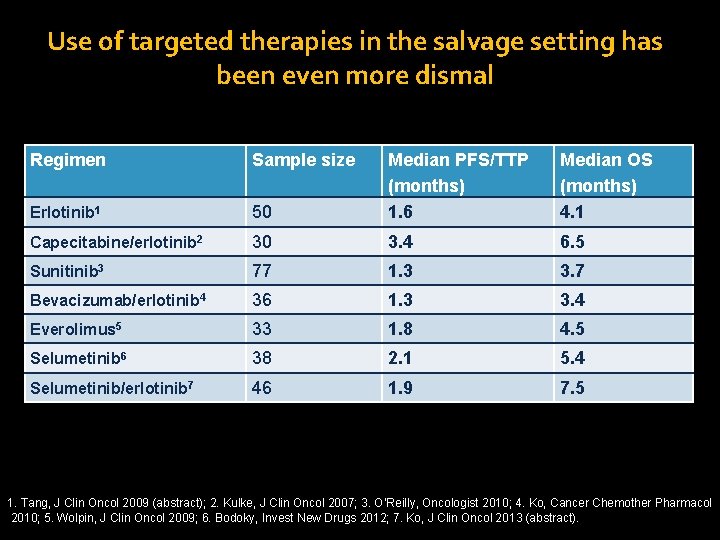
Use of targeted therapies in the salvage setting has been even more dismal Regimen Sample size 50 Median PFS/TTP (months) 1. 6 Median OS (months) 4. 1 Erlotinib 1 Capecitabine/erlotinib 2 30 3. 4 6. 5 Sunitinib 3 77 1. 3 3. 7 Bevacizumab/erlotinib 4 36 1. 3 3. 4 Everolimus 5 33 1. 8 4. 5 Selumetinib 6 38 2. 1 5. 4 Selumetinib/erlotinib 7 46 1. 9 7. 5 1. Tang, J Clin Oncol 2009 (abstract); 2. Kulke, J Clin Oncol 2007; 3. O’Reilly, Oncologist 2010; 4. Ko, Cancer Chemother Pharmacol 2010; 5. Wolpin, J Clin Oncol 2009; 6. Bodoky, Invest New Drugs 2012; 7. Ko, J Clin Oncol 2013 (abstract).

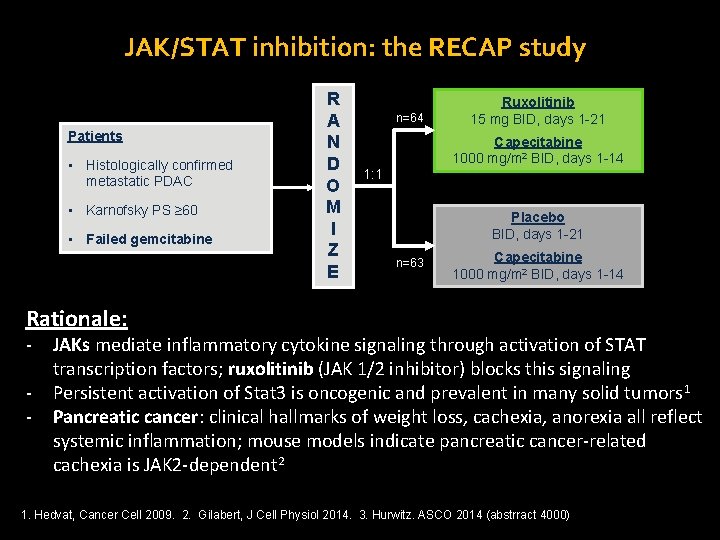
JAK/STAT inhibition: the RECAP study Patients • Histologically confirmed metastatic PDAC • Karnofsky PS ≥ 60 • Failed gemcitabine R A N D O M I Z E n=64 Ruxolitinib 15 mg BID, days 1 -21 Capecitabine 1000 mg/m 2 BID, days 1 -14 1: 1 Placebo BID, days 1 -21 n=63 Capecitabine 1000 mg/m 2 BID, days 1 -14 Rationale: - JAKs mediate inflammatory cytokine signaling through activation of STAT transcription factors; ruxolitinib (JAK 1/2 inhibitor) blocks this signaling Persistent activation of Stat 3 is oncogenic and prevalent in many solid tumors 1 Pancreatic cancer: clinical hallmarks of weight loss, cachexia, anorexia all reflect systemic inflammation; mouse models indicate pancreatic cancer-related cachexia is JAK 2 -dependent 2 1. Hedvat, Cancer Cell 2009. 2. Gilabert, J Cell Physiol 2014. 3. Hurwitz. ASCO 2014 (abstrract 4000)

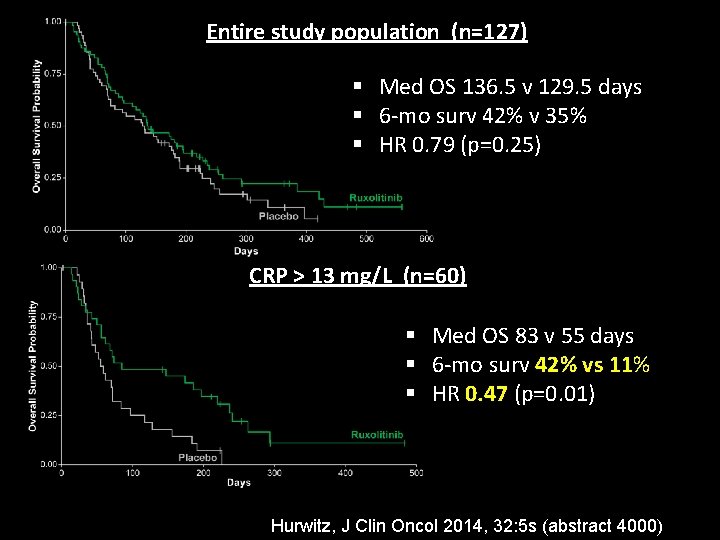
Entire study population (n=127) § Med OS 136. 5 v 129. 5 days § 6 -mo surv 42% v 35% § HR 0. 79 (p=0. 25) CRP > 13 mg/L (n=60) § Med OS 83 v 55 days § 6 -mo surv 42% vs 11% § HR 0. 47 (p=0. 01) Hurwitz, J Clin Oncol 2014, 32: 5 s (abstract 4000)

Recapping RECAP • Survival benefit of ruxolitinib may relate to alleviating cachexia and inanition, as much as reducing tumor burden • Ongoing JANUS-1 and -2 trials (registrational phase III studies) selected specifically for patients with high levels of C-reactive protein (biomarker-driven) • Is capecitabine alone as a reference arm adequate in the second-line setting?

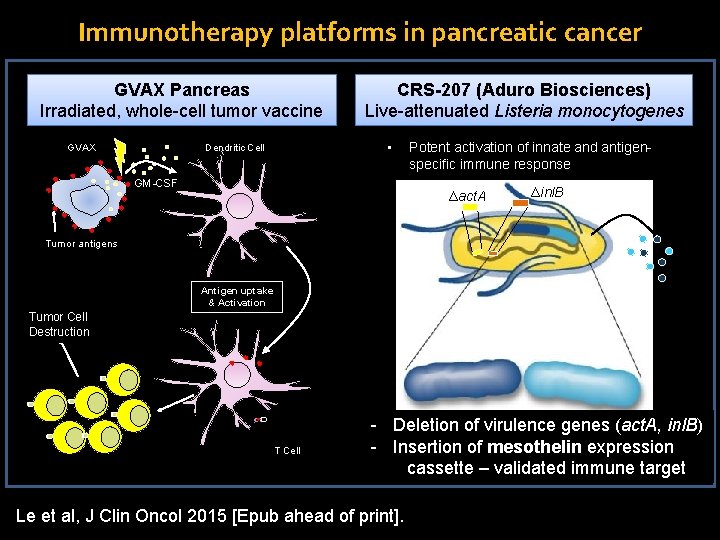
Immunotherapy platforms in pancreatic cancer GVAX Pancreas Irradiated, whole-cell tumor vaccine GVAX CRS-207 (Aduro Biosciences) Live-attenuated Listeria monocytogenes • Dendritic Cell GM-CSF Potent activation of innate and antigenspecific immune response Δact. A Δinl. B Tumor antigens Antigen uptake & Activation Tumor Cell Destruction T Cell - Deletion of virulence genes (act. A, inl. B) - Insertion of mesothelin expression cassette – validated immune target Le et al, J Clin Oncol 2015 [Epub ahead of print].

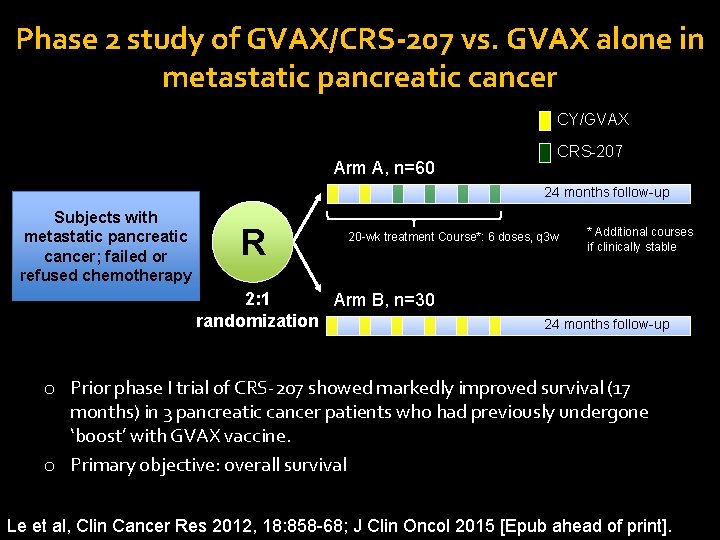
Phase 2 study of GVAX/CRS-207 vs. GVAX alone in metastatic pancreatic cancer CY/GVAX Arm A, n=60 CRS-207 24 months follow-up Subjects with metastatic pancreatic cancer; failed or refused chemotherapy R R 20 -wk treatment Course*: 6 doses, q 3 w 2: 1 Arm B, n=30 randomization * Additional courses if clinically stable 24 months follow-up o Prior phase I trial of CRS-207 showed markedly improved survival (17 months) in 3 pancreatic cancer patients who had previously undergone ‘boost’ with GVAX vaccine. o Primary objective: overall survival Le et al, Clin Cancer Res 2012, 18: 858 -68; J Clin Oncol 2015 [Epub ahead of print].

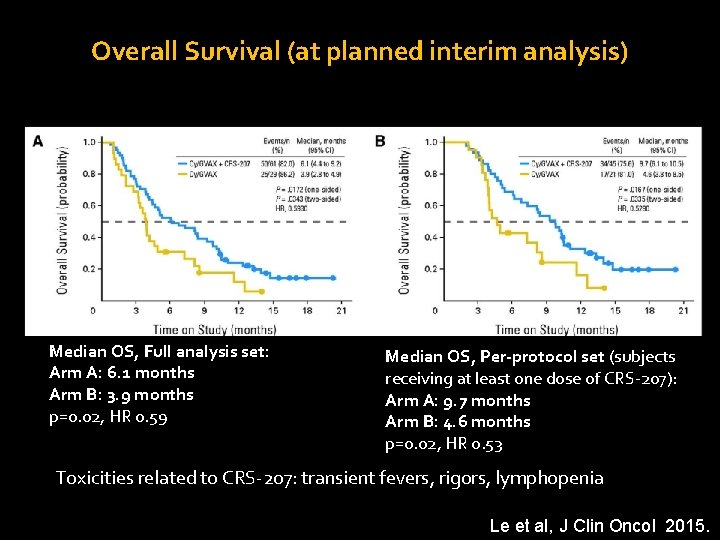
Overall Survival (at planned interim analysis) Median OS, Full analysis set: Arm A: 6. 1 months Arm B: 3. 9 months p=0. 02, HR 0. 59 Median OS, Per-protocol set (subjects receiving at least one dose of CRS-207): Arm A: 9. 7 months Arm B: 4. 6 months p=0. 02, HR 0. 53 Toxicities related to CRS-207: transient fevers, rigors, lymphopenia Le et al, J Clin Oncol 2015.

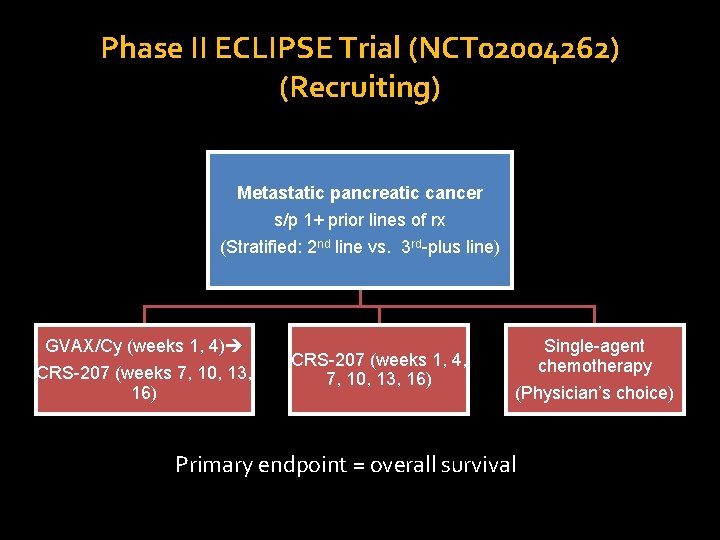
Phase II ECLIPSE Trial (NCT 02004262) (Recruiting) Metastatic pancreatic cancer s/p 1+ prior lines of rx (Stratified: 2 nd line vs. 3 rd-plus line) GVAX/Cy (weeks 1, 4) CRS-207 (weeks 7, 10, 13, 16) CRS-207 (weeks 1, 4, 7, 10, 13, 16) Single-agent chemotherapy (Physician’s choice) Primary endpoint = overall survival

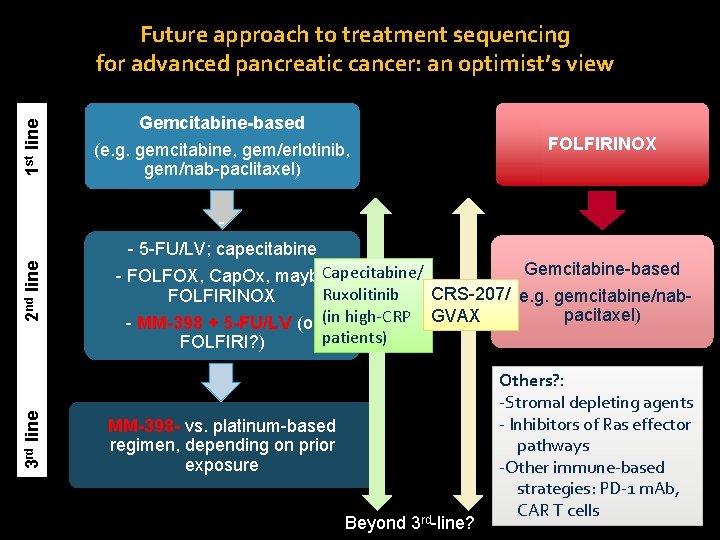
1 st line Future approach to treatment sequencing for advanced pancreatic cancer: an optimist’s view Gemcitabine-based (e. g. gemcitabine, gem/erlotinib, gem/nab-paclitaxel) FOLFIRINOX 2 nd line - 5 -FU/LV; capecitabine Gemcitabine-based - FOLFOX, Cap. Ox, maybe. Capecitabine/ Ruxolitinib CRS-207/ (e. g. gemcitabine/nab. FOLFIRINOX pacitaxel) - MM-398 + 5 -FU/LV (or (in high-CRP GVAX patients) FOLFIRI? ) 3 rd line - Others? : -Stromal depleting agents - Inhibitors of Ras effector pathways -Other immune-based strategies: PD-1 m. Ab, CAR T cells MM-398 - vs. platinum-based regimen, depending on prior exposure Beyond 3 rd-line?