Treatment algorithm for MBC HR HER 2 SERM

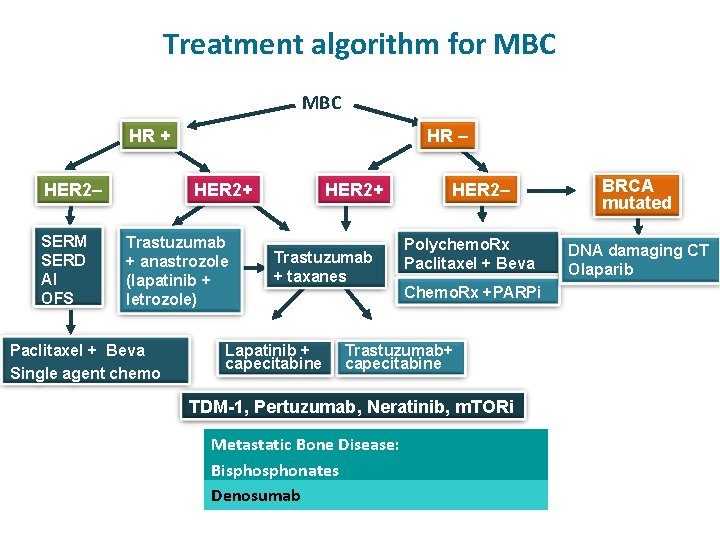
Treatment algorithm for MBC HR + HER 2– SERM SERD AI OFS HR – Trastuzumab + anastrozole (lapatinib + letrozole) Paclitaxel + Beva Single agent chemo HER 2+ Trastuzumab + taxanes Lapatinib + capecitabine HER 2– Polychemo. Rx Paclitaxel + Beva Chemo. Rx +PARPi Trastuzumab+ capecitabine TDM-1, Pertuzumab, Neratinib, m. TORi Metastatic Bone Disease: Bisphonates Denosumab BRCA mutated DNA damaging CT Olaparib
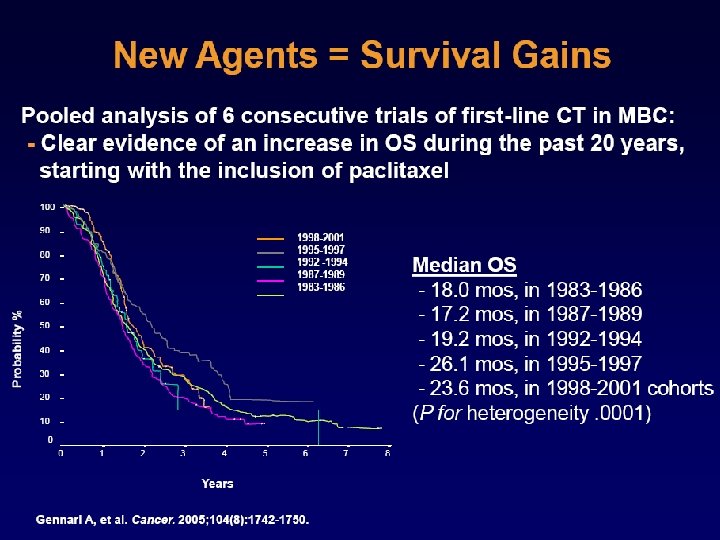

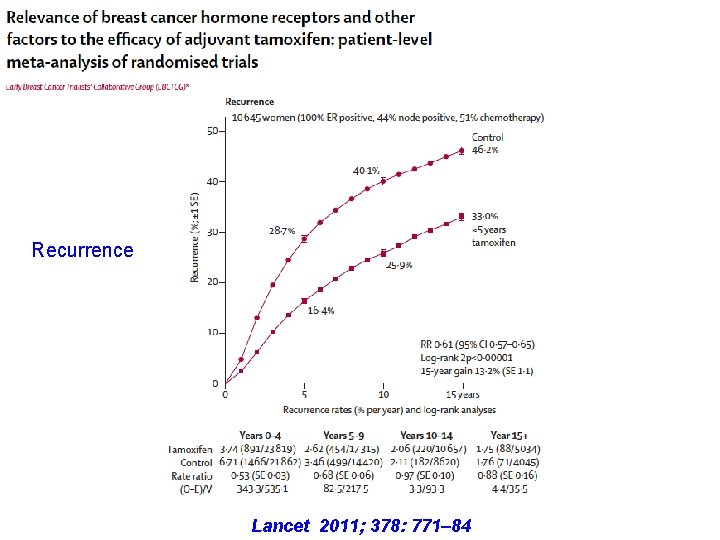
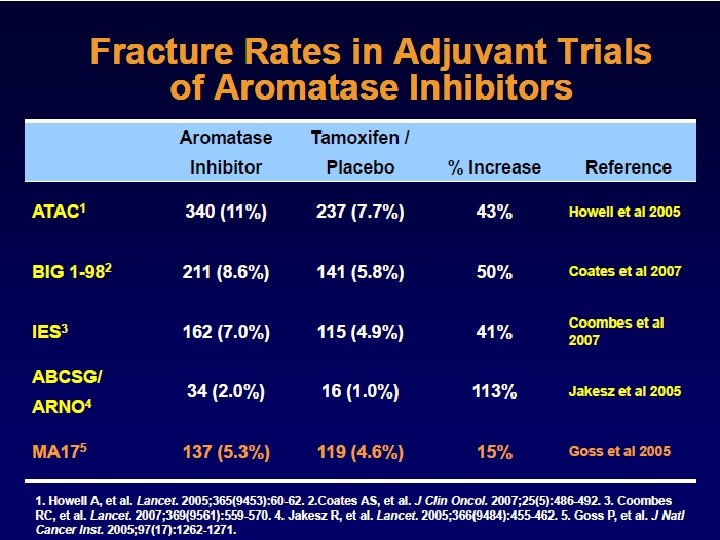
Recurrence Lancet 2011; 378: 771– 84
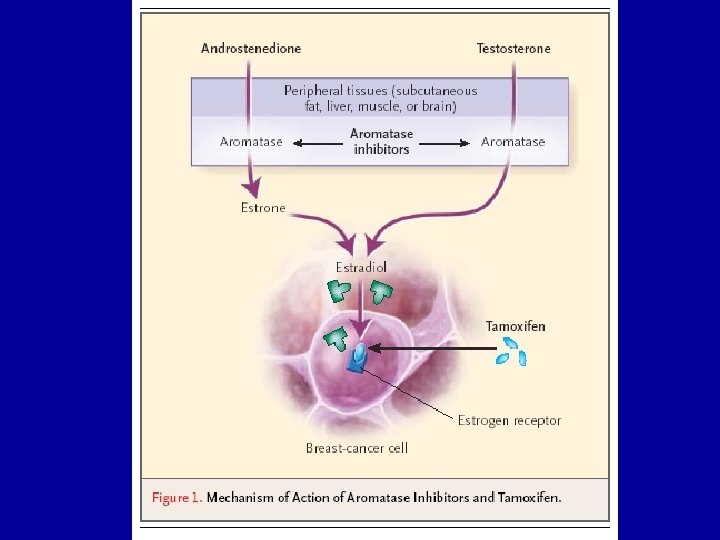

Lancet Oncol. 2010 Dec; 11(12): 1135 -41
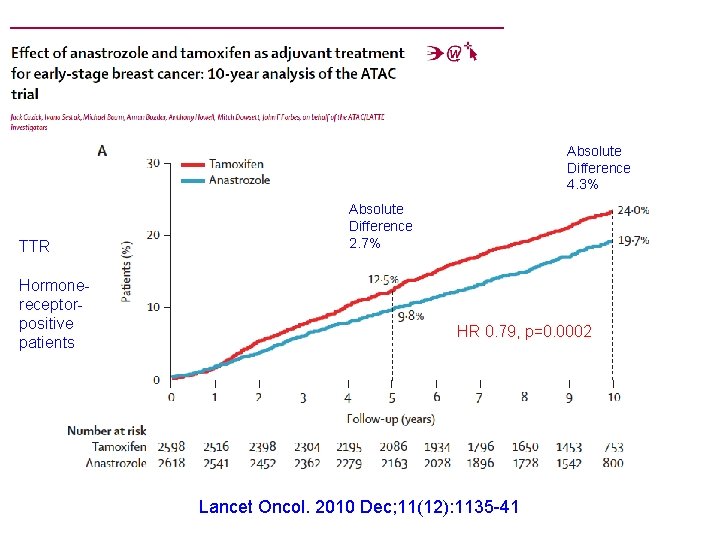
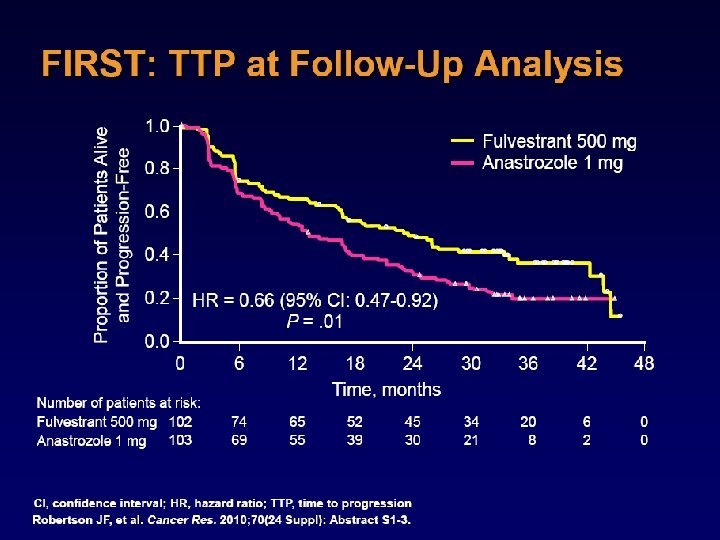
Absolute Difference 4. 3% TTR Hormonereceptorpositive patients Absolute Difference 2. 7% HR 0. 79, p=0. 0002 Lancet Oncol. 2010 Dec; 11(12): 1135 -41
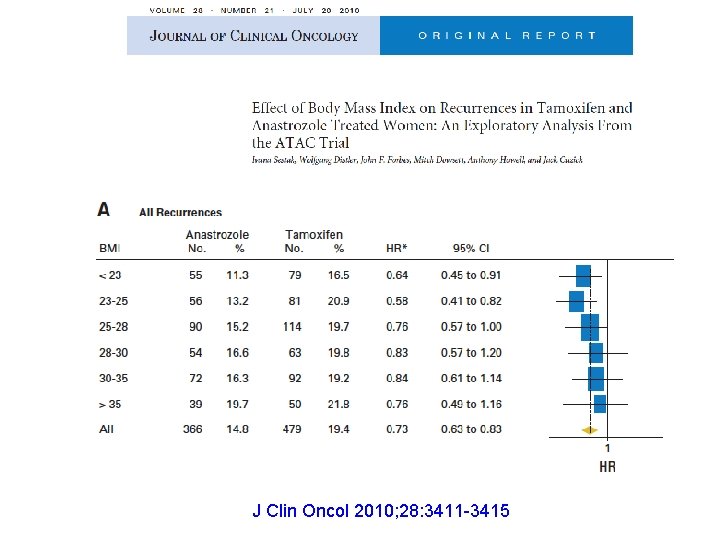
J Clin Oncol 2010; 28: 3411 -3415
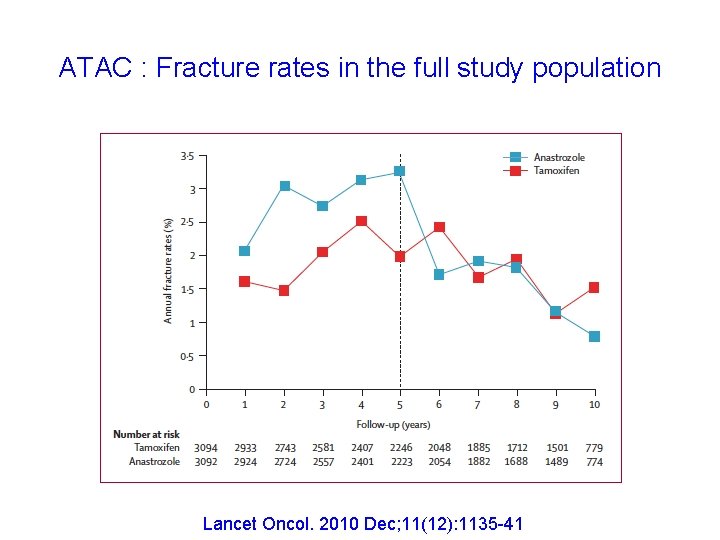
ATAC : Fracture rates in the full study population Lancet Oncol. 2010 Dec; 11(12): 1135 -41
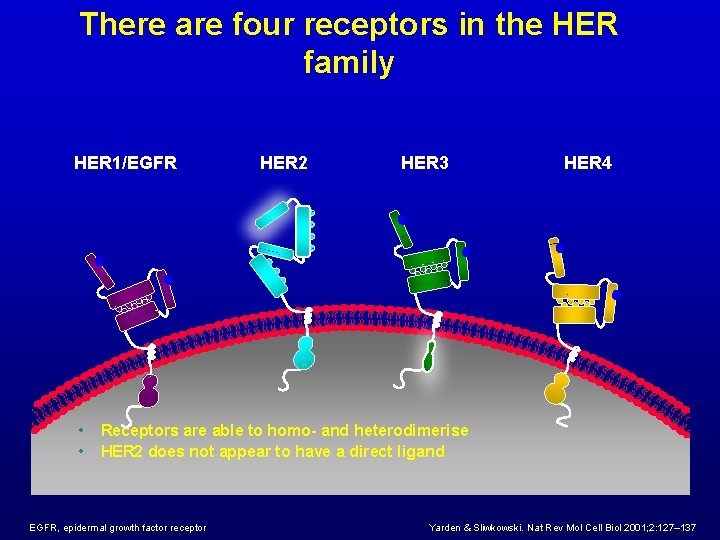
There are four receptors in the HER family HER 1/EGFR • • HER 2 HER 3 HER 4 Receptors are able to homo- and heterodimerise HER 2 does not appear to have a direct ligand EGFR, epidermal growth factor receptor Yarden & Sliwkowski. Nat Rev Mol Cell Biol 2001; 2: 127– 137
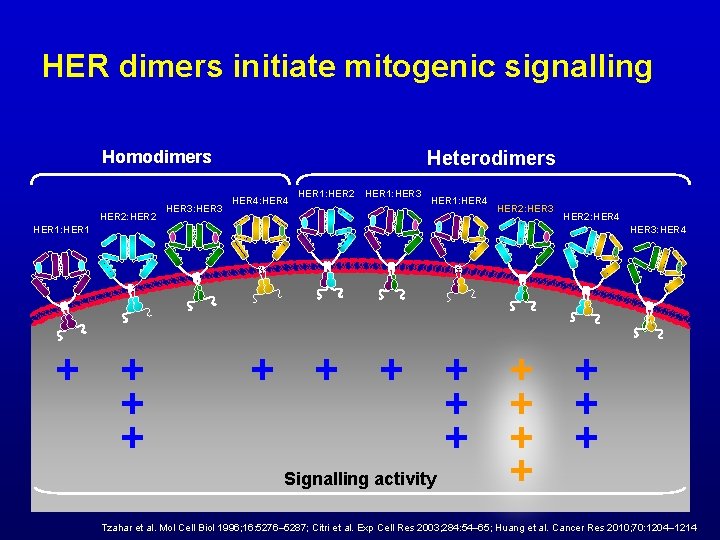
HER dimers initiate mitogenic signalling Homodimers HER 2: HER 2 HER 3: HER 3 Heterodimers HER 4: HER 4 HER 1: HER 2 HER 1: HER 3 HER 1: HER 4 HER 2: HER 3 HER 2: HER 4 HER 1: HER 1 HER 3: HER 4 + + + + Signalling activity + Tzahar et al. Mol Cell Biol 1996; 16: 5276– 5287; Citri et al. Exp Cell Res 2003; 284: 54– 65; Huang et al. Cancer Res 2010; 70: 1204– 1214

Trastuzumab targets HER 2 and Lapatinib targets both EGFR and HER 2 The HER 2 gene is localized to chromosome 17 q l HER 2 and EGFR are tyrosine kinase transmembrane growth factor receptors l Fernandes et al, Cancer Lett 1999; Moghal et al, Curr Opin Cell Biol 1999; Yarden et al, Nat Rev Mol Cell Biol 2001
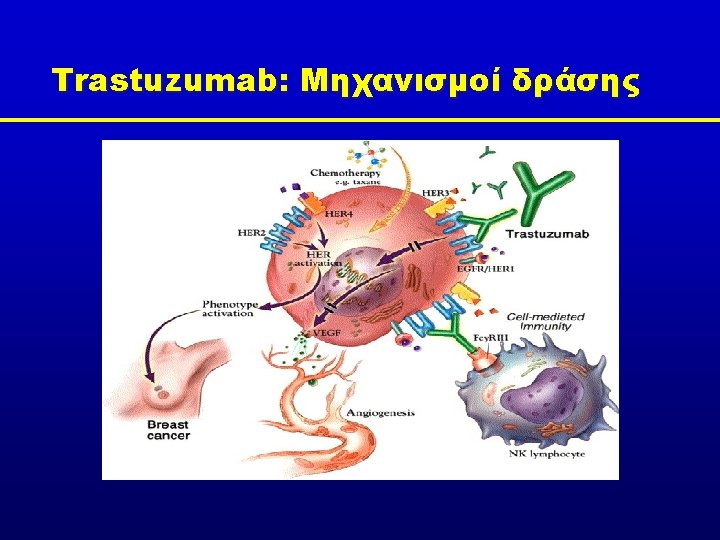
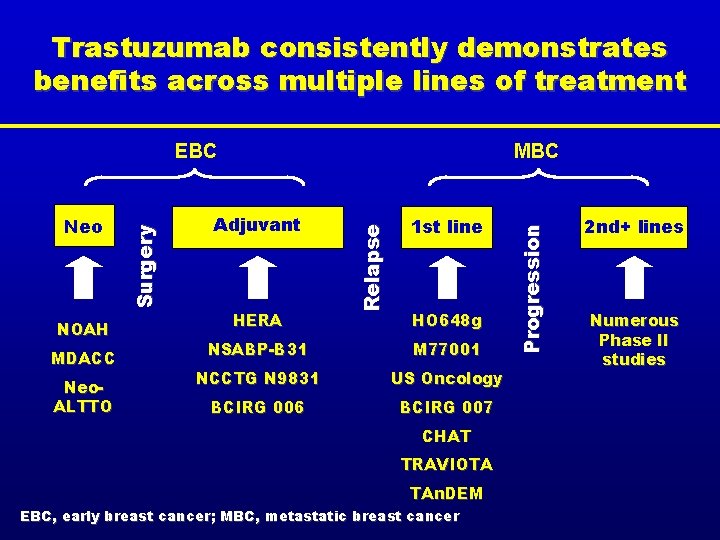
Trastuzumab consistently demonstrates benefits across multiple lines of treatment MDACC Neo. ALTTO Adjuvant HERA 1 st line HO 648 g NSABP-B 31 M 77001 NCCTG N 9831 US Oncology BCIRG 006 BCIRG 007 CHAT TRAVIOTA TAn. DEM EBC, early breast cancer; MBC, metastatic breast cancer P r o g r e s s io n NOAH MBC R e la p s e Neo Su r g e r y EBC 2 nd+ lines Numerous Phase II studies
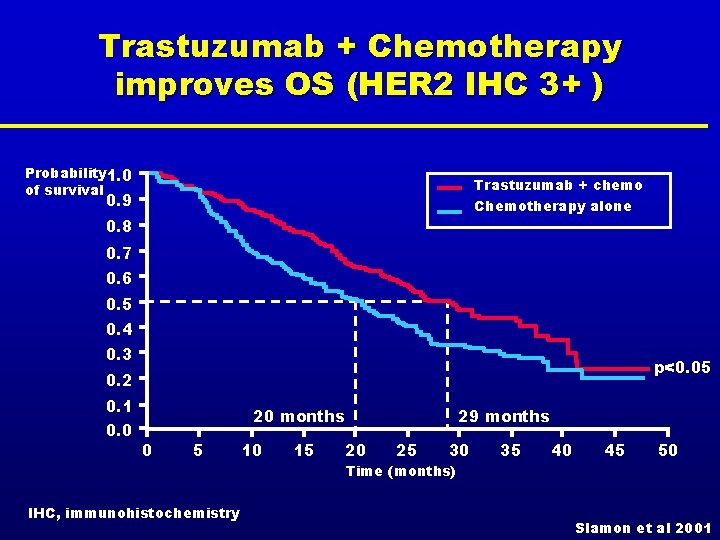
Trastuzumab + Chemotherapy improves OS (HER 2 IHC 3+ ) Probability 1. 0 of survival Trastuzumab + chemo Chemotherapy alone 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 p<0. 05 0. 2 0. 1 0. 0 20 months 0 5 IHC, immunohistochemistry 10 15 29 months 20 25 30 Time (months) 35 40 45 50 Slamon et al 2001
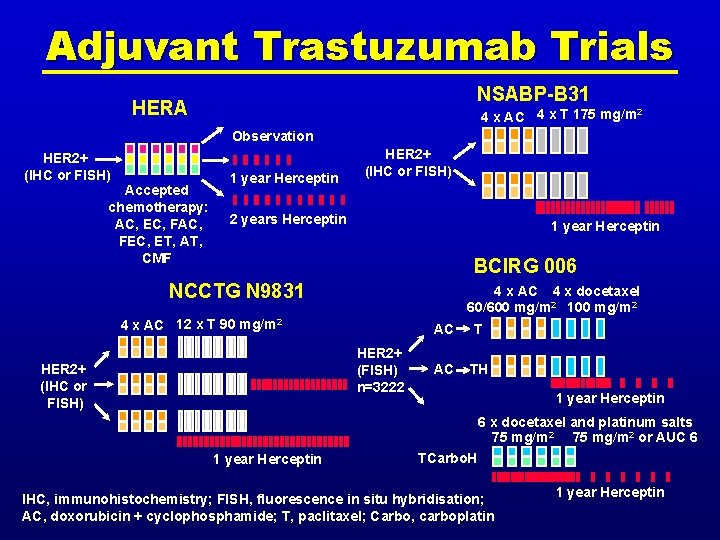
Adjuvant Trastuzumab Trials NSABP-B 31 HERA 4 x AC 4 x T 175 mg/m 2 Observation HER 2+ (IHC or FISH) Accepted chemotherapy: AC, EC, FAC, FEC, ET, AT, CMF 1 year Herceptin HER 2+ (IHC or FISH) 2 years Herceptin 1 year Herceptin BCIRG 006 NCCTG N 9831 4 x AC 4 x docetaxel 60/600 mg/m 2 100 mg/m 2 4 x AC 12 x T 90 mg/m 2 HER 2+ (FISH) n=3222 HER 2+ (IHC or FISH) AC TH 1 year Herceptin 6 x docetaxel and platinum salts 75 mg/m 2 or AUC 6 1 year Herceptin TCarbo. H IHC, immunohistochemistry; FISH, fluorescence in situ hybridisation; AC, doxorubicin + cyclophosphamide; T, paclitaxel; Carbo, carboplatin 1 year Herceptin
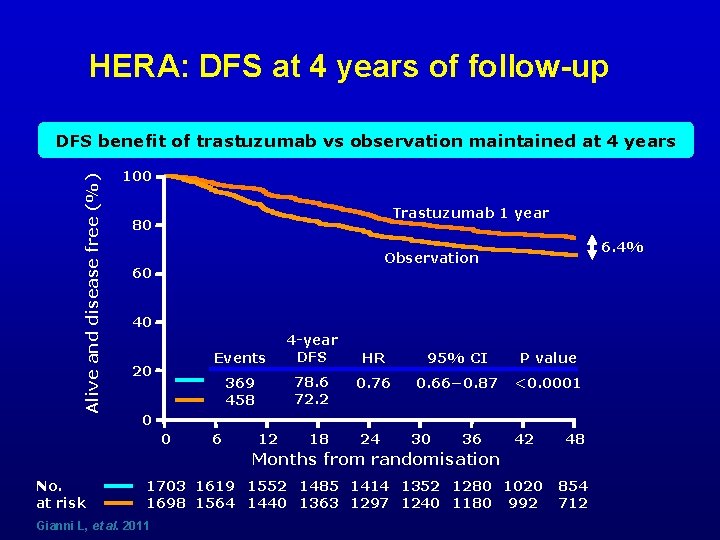
HERA: DFS at 4 years of follow-up Alive and disease free (%) DFS benefit of trastuzumab vs observation maintained at 4 years 100 Trastuzumab 1 year 80 6. 4% Observation 60 40 20 Events 4 -year DFS 369 458 78. 6 72. 2 HR 95% CI P value 0. 76 0. 66− 0. 87 <0. 0001 0 0 6 12 18 24 30 36 42 48 Months from randomisation No. at risk 1703 1619 1552 1485 1414 1352 1280 1020 1698 1564 1440 1363 1297 1240 1180 992 Gianni L, et al. 2011 854 712
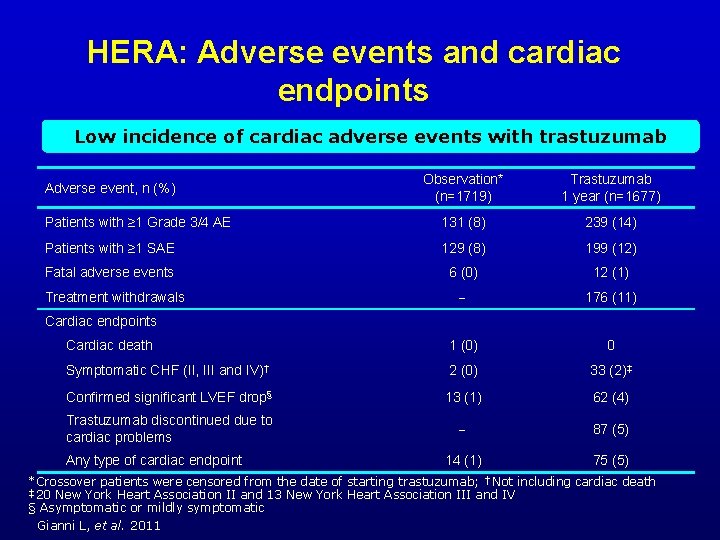
HERA: Adverse events and cardiac endpoints Low incidence of cardiac adverse events with trastuzumab Observation* (n=1719) Trastuzumab 1 year (n=1677) Patients with ≥ 1 Grade 3/4 AE 131 (8) 239 (14) Patients with ≥ 1 SAE 129 (8) 199 (12) Fatal adverse events 6 (0) 12 (1) 176 (11) Cardiac death 1 (0) 0 Symptomatic CHF (II, III and IV)† 2 (0) 33 (2)‡ Confirmed significant LVEF drop§ 13 (1) 62 (4) Trastuzumab discontinued due to cardiac problems 87 (5) 14 (1) 75 (5) Adverse event, n (%) Treatment withdrawals Cardiac endpoints Any type of cardiac endpoint *Crossover patients were censored from the date of starting trastuzumab; †Not including cardiac death ‡ 20 New York Heart Association II and 13 New York Heart Association III and IV § Asymptomatic or mildly symptomatic Gianni L, et al. 2011
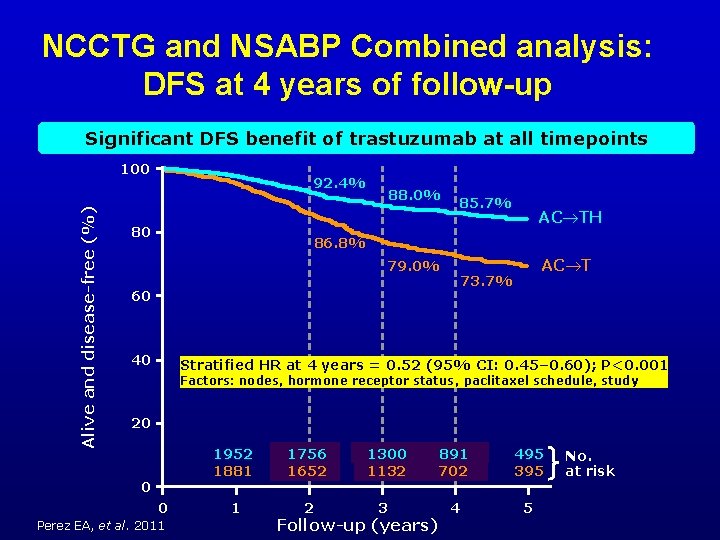
NCCTG and NSABP Combined analysis: DFS at 4 years of follow-up Significant DFS benefit of trastuzumab at all timepoints Alive and disease-free (%) 100 92. 4% 80 88. 0% 85. 7% AC TH 86. 8% 79. 0% 73. 7% 60 40 AC T Stratified HR at 4 years = 0. 52 (95% CI: 0. 45‒ 0. 60); P<0. 001 Factors: nodes, hormone receptor status, paclitaxel schedule, study 20 0 0 Perez EA, et al. 2011 1952 1881 1756 1652 1 2 1300 1132 3 Follow-up (years) 891 702 495 395 4 5 No. at risk
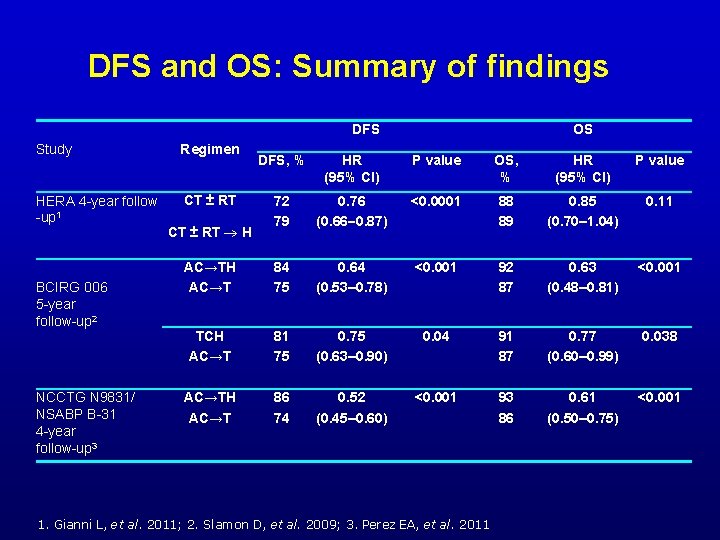
DFS and OS: Summary of findings DFS Study Regimen HERA 4 -year follow -up 1 CT ± RT BCIRG 006 5 -year follow-up 2 NCCTG N 9831/ NSABP B-31 4 -year follow-up 3 OS DFS, % HR (95% CI) P value OS, % HR (95% CI) P value 72 79 0. 76 (0. 66 0. 87) <0. 0001 88 89 0. 85 (0. 70– 1. 04) 0. 11 AC→TH AC→T 84 75 0. 64 (0. 53– 0. 78) <0. 001 92 87 0. 63 (0. 48– 0. 81) <0. 001 TCH AC→T 81 75 0. 75 (0. 63– 0. 90) 0. 04 91 87 0. 77 (0. 60– 0. 99) 0. 038 AC→TH AC→T 86 74 0. 52 (0. 45– 0. 60) <0. 001 93 86 0. 61 (0. 50– 0. 75) <0. 001 CT ± RT H 1. Gianni L, et al. 2011; 2. Slamon D, et al. 2009; 3. Perez EA, et al. 2011
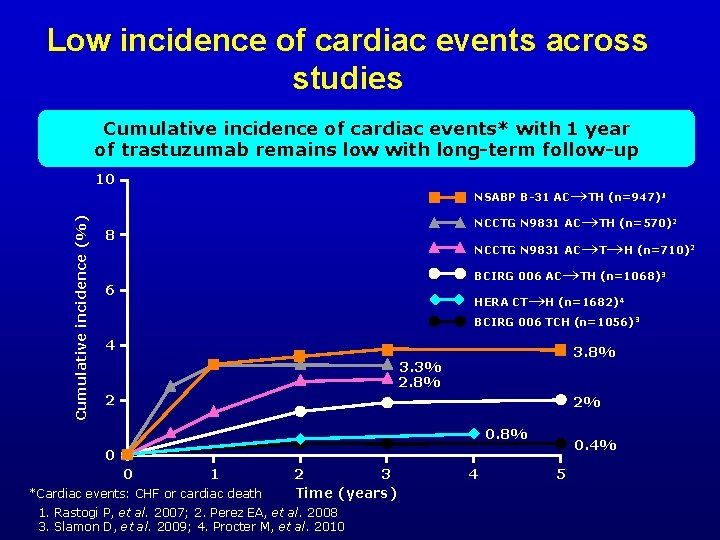
Low incidence of cardiac events across studies Cumulative incidence of cardiac events* with 1 year of trastuzumab remains low with long-term follow-up 10 TH (n=947)1 NCCTG N 9831 AC TH (n=570)2 NCCTG N 9831 AC T H (n=710)2 BCIRG 006 AC TH (n=1068) 3 HERA CT H (n=1682) 4 Cumulative incidence (%) NSABP B-31 AC 8 6 BCIRG 006 TCH (n=1056) 3 4 3. 8% 3. 3% 2. 8% 2 2% 0. 8% 0. 4% 0 0 1 *Cardiac events: CHF or cardiac death 2 3 Time (years) 1. Rastogi P, et al. 2007; 2. Perez EA, et al. 2008 3. Slamon D, et al. 2009; 4. Procter M, et al. 2010 4 5
Conclusions Trastuzumab for 1 year for patients with HER 2 -positive EBC • DFS and OS benefits with trastuzumab are maintained at long-term follow-up – Trastuzumab may be administered either concurrently or sequentially with chemotherapy – Trastuzumab is well tolerated with a consistent safety and tolerability profile – Incidence of cardiac events remains low
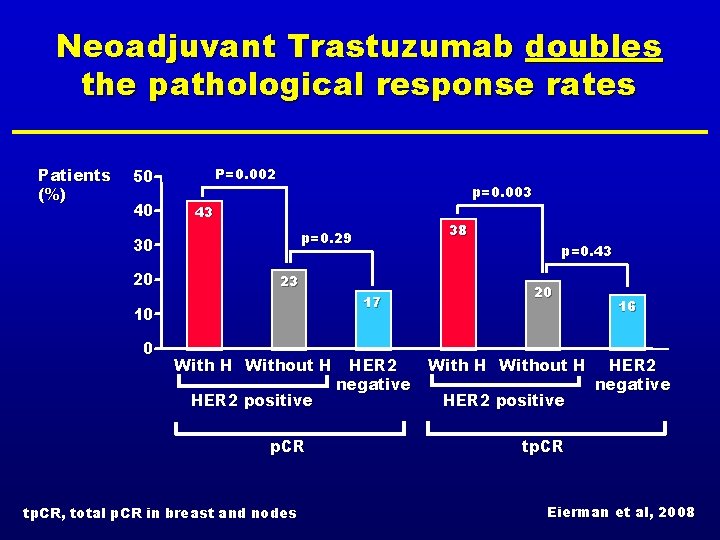
Neoadjuvant Trastuzumab doubles the pathological response rates Patients (%) P=0. 002 50 40 p=0. 003 43 30 20 p=0. 43 23 17 10 0 38 p=0. 29 With H Without H HER 2 positive p. CR tp. CR, total p. CR in breast and nodes HER 2 negative 20 With H Without H HER 2 positive 16 HER 2 negative tp. CR Eierman et al, 2008
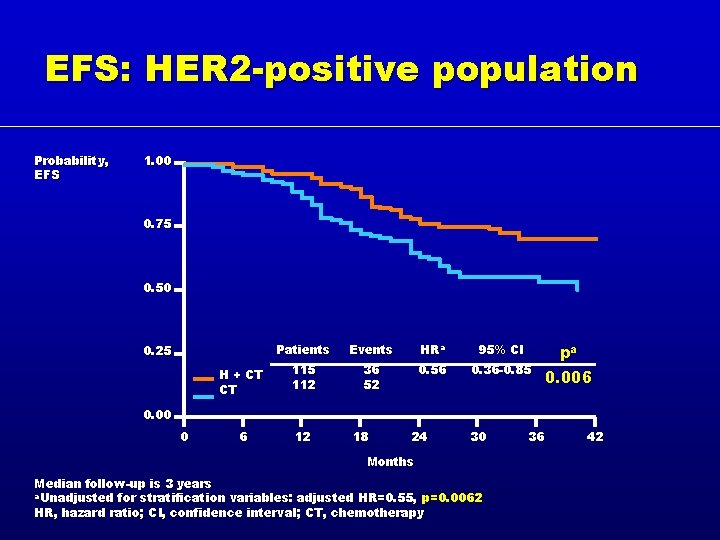
EFS: HER 2 -positive population Probability, EFS 1. 00 0. 75 0. 50 Patients Events HRa 95% CI H + CT CT 115 112 36 52 0. 56 0. 36 -0. 85 6 12 0. 25 pa 0. 006 0. 00 0 18 24 30 Months Median follow-up is 3 years a. Unadjusted for stratification variables: adjusted HR=0. 55, p=0. 0062 HR, hazard ratio; CI, confidence interval; CT, chemotherapy 36 42
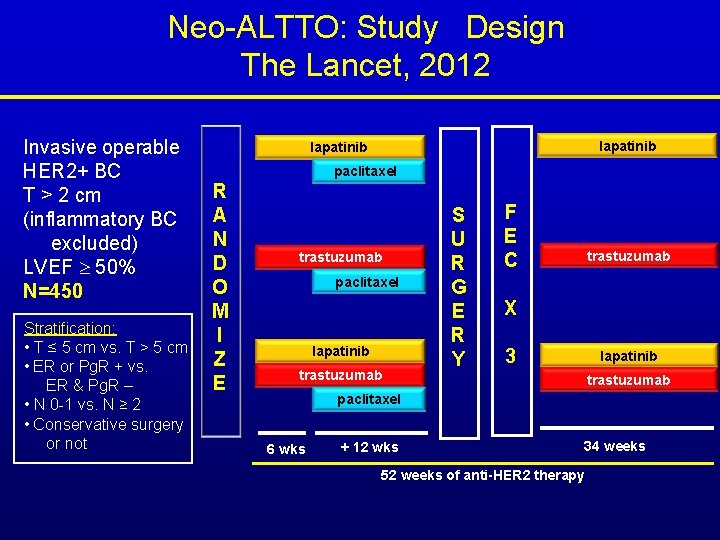
Neo-ALTTO: Study Design The Lancet, 2012 Invasive operable HER 2+ BC T > 2 cm (inflammatory BC excluded) LVEF 50% N=450 Stratification: • T ≤ 5 cm vs. T > 5 cm • ER or Pg. R + vs. ER & Pg. R – • N 0 -1 vs. N ≥ 2 • Conservative surgery or not lapatinib paclitaxel R A N D O M I Z E trastuzumab paclitaxel lapatinib trastuzumab S U R G E R Y F E C trastuzumab X 3 lapatinib trastuzumab paclitaxel 6 wks + 12 wks 34 weeks 52 weeks of anti-HER 2 therapy

Neo-ALTTO: Efficacy – p. CR and tp. CR The Lancet, 2012 L: lapatinib; T: trastuzumab; L+T: lapatinib plus trastuzumab p. CR pathologic complete response

Conclusions • Primary objective was achieved for the combination arm: 51. 3% p. CR rate (L+T) was significantly higher than 29. 5% (T) • There was an increased toxicity, but manageable, observed in the lapatinib arms (diarrhea and liver enzyme alterations)

Future Directions
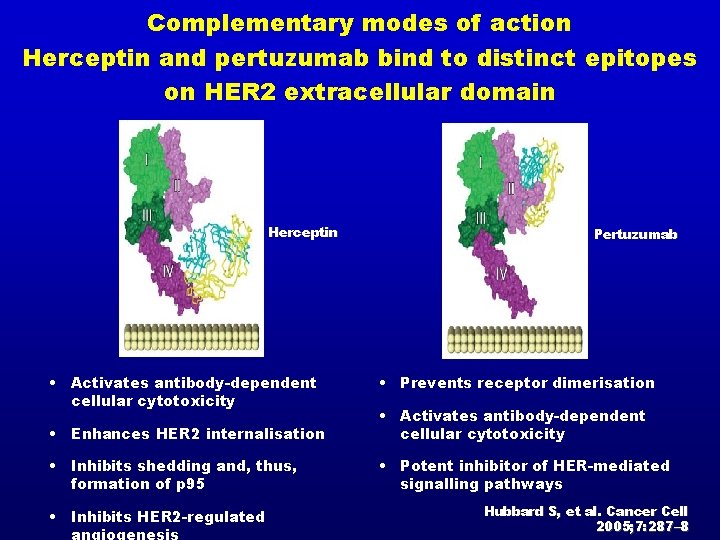
Complementary modes of action Herceptin and pertuzumab bind to distinct epitopes on HER 2 extracellular domain Herceptin • Activates antibody-dependent cellular cytotoxicity Pertuzumab • Prevents receptor dimerisation • Enhances HER 2 internalisation • Activates antibody-dependent cellular cytotoxicity • Inhibits shedding and, thus, formation of p 95 • Potent inhibitor of HER-mediated signalling pathways • Inhibits HER 2 -regulated angiogenesis Hubbard S, et al. Cancer Cell 2005; 7: 287– 8

Overall survival: Predefined interim analysis Median follow-up: 19. 3 months, n = 165 OS events 100 Overall survival (%) 90 80 70 HR = 0. 64* 95% CI 0. 47‒ 0. 88 p = 0. 0053* 60 50 40 30 Pertuzumab + Trast + Doc: 69 events 20 Placebo + Trast + Doc: 96 events 10 0 0 5 10 15 20 25 30 35 40 45 Time (months) n at risk Pertuzumab + T + D 402 387 367 251 161 87 31 4 0 0 Placebo + T + D 383 347 228 143 67 24 2 0 0 406 * The interim OS analysis did not cross the pre-specified O’Brien-Fleming stopping boundary (HR ≤ 0. 603; p ≤ 0. 0012) Baselga et al SABCS 2011
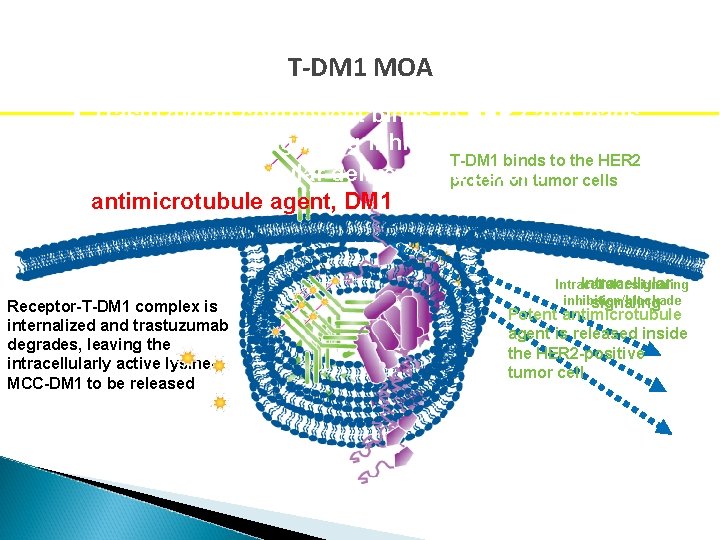
T-DM 1 MOA • Trastuzumab component binds to HER 2 and leads to downstream signaling inhibition/blockade T-DM 1 binds to the HER 2 • Targeted intracellular delivery of protein a potent on tumor cells antimicrotubule agent, DM 1 Receptor-T-DM 1 complex is internalized and trastuzumab degrades, leaving the intracellularly active lysine. MCC-DM 1 to be released Intracellular signaling Intracellular inhibition/blockade signaling Potent antimicrotubule agent is released inside the HER 2 -positive tumor cell

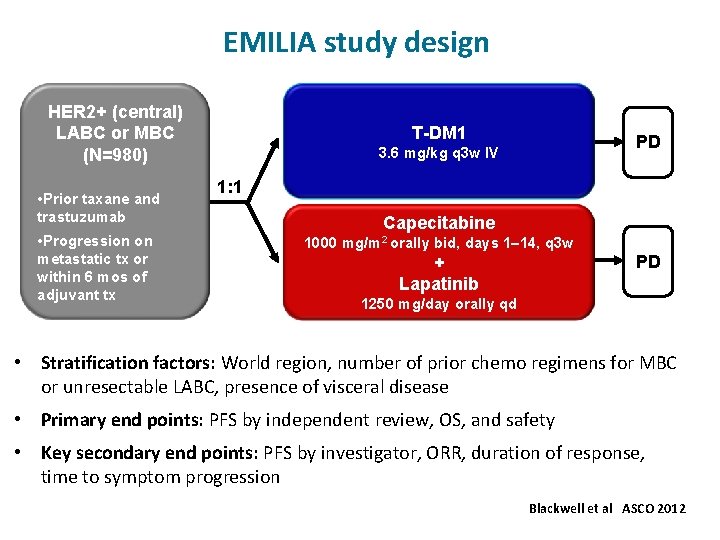
EMILIA study design HER 2+ (central) LABC or MBC (N=980) • Prior taxane and trastuzumab • Progression on metastatic tx or within 6 mos of adjuvant tx T-DM 1 PD 3. 6 mg/kg q 3 w IV 1: 1 Capecitabine 1000 mg/m 2 orally bid, days 1– 14, q 3 w PD + Lapatinib 1250 mg/day orally qd • Stratification factors: World region, number of prior chemo regimens for MBC or unresectable LABC, presence of visceral disease • Primary end points: PFS by independent review, OS, and safety • Key secondary end points: PFS by investigator, ORR, duration of response, time to symptom progression Blackwell et al ASCO 2012
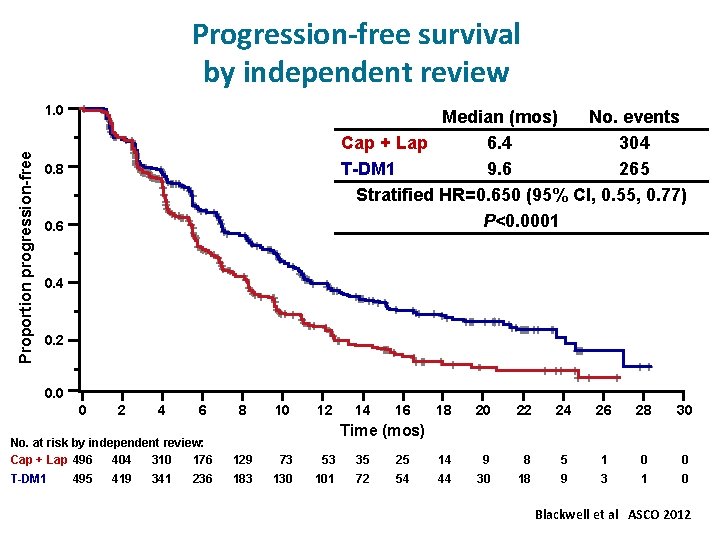
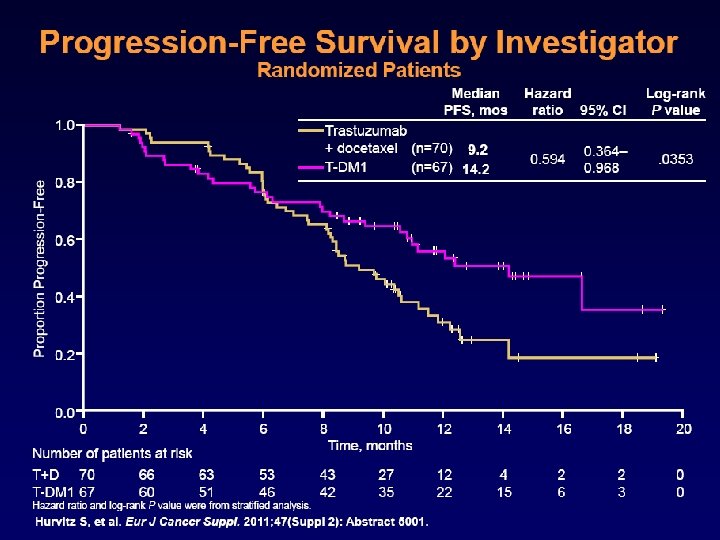
Progression-free survival by independent review Proportion progression-free 1. 0 Median (mos) No. events Cap + Lap 6. 4 304 T-DM 1 9. 6 265 Stratified HR=0. 650 (95% CI, 0. 55, 0. 77) P<0. 0001 0. 8 0. 6 0. 4 0. 2 0. 0 0 2 4 6 No. at risk by independent review: 404 310 176 Cap + Lap 496 T-DM 1 495 419 341 236 Unstratified HR=0. 66 (P<0. 0001). 8 10 12 14 16 18 20 22 24 26 28 30 Time (mos) 129 73 53 35 25 14 9 8 5 1 0 0 183 130 101 72 54 44 30 18 9 3 1 0 Blackwell et al ASCO 2012
The Revolution of Molecular Targeted Cancer Therapy Trastuzumab Pertuzumab Lapatinib TDM-1 Ben-Kasus et al. , Molecular Oncology
- Slides: 48