TRATAMIENTO SISTMICO EN TNE AVANZADOS SECUENCIAS DE TRATAMIENTO






- Slides: 34

TRATAMIENTO SISTÉMICO EN TNE AVANZADOS: SECUENCIAS DE TRATAMIENTO Y SELECCIÓN DE PACIENTES Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d’Hebron University Hospital Vall d’Hebron Institute of Oncology Barcelona

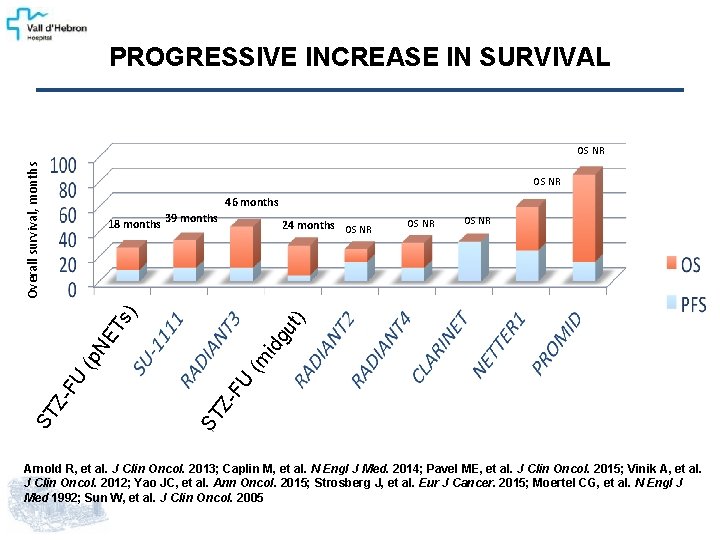
PROGRESSIVE INCREASE IN SURVIVAL Overall survival, months OS NR 46 months OS NR (m idg ut NE T ST ZFU (p FU ST Z- 24 months OS NR ) s) 18 months 39 months Arnold R, et al. J Clin Oncol. 2013; Caplin M, et al. N Engl J Med. 2014; Pavel ME, et al. J Clin Oncol. 2015; Vinik A, et al. J Clin Oncol. 2012; Yao JC, et al. Ann Oncol. 2015; Strosberg J, et al. Eur J Cancer. 2015; Moertel CG, et al. N Engl J Med 1992; Sun W, et al. J Clin Oncol. 2005

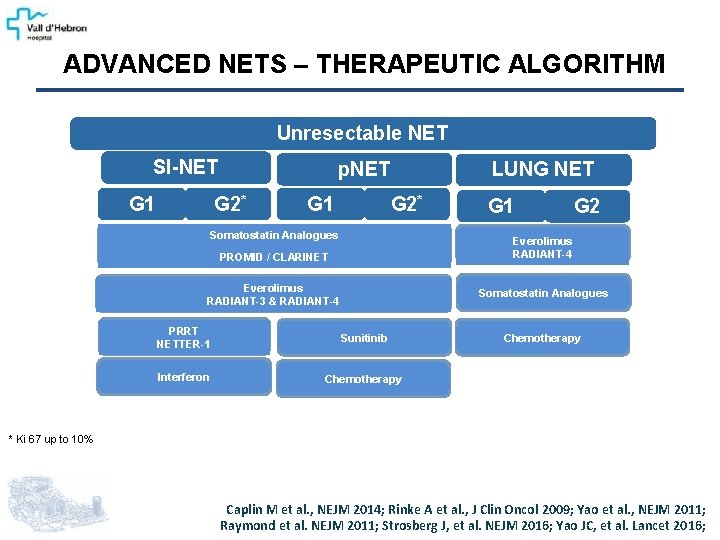
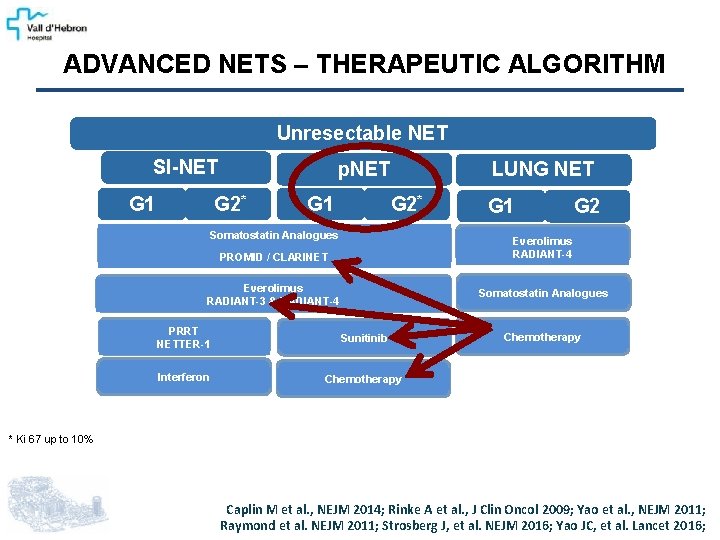
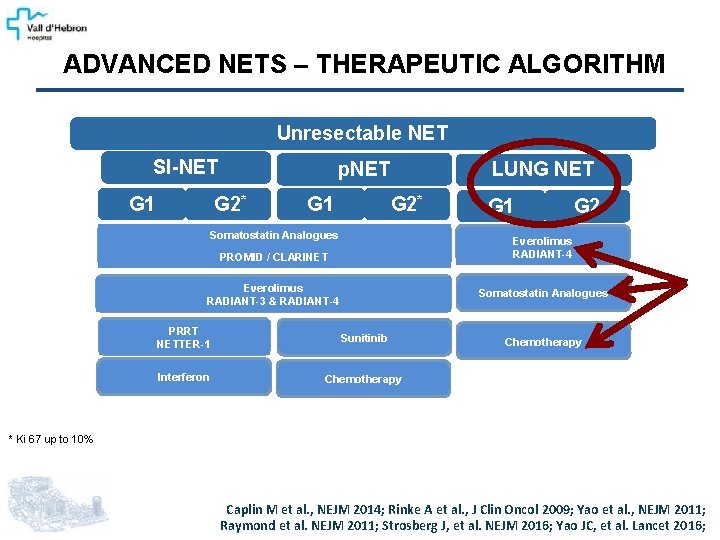
ADVANCED NETS – THERAPEUTIC ALGORITHM Unresectable NET SI-NET G 1 p. NET G 2* G 1 LUNG NET G 2* Somatostatin Analogues G 1 G 2 Everolimus RADIANT-4 PROMID / CLARINET Everolimus RADIANT-3 & RADIANT-4 Somatostatin Analogues PRRT NETTER-1 Sunitinib Interferon Chemotherapy * Ki 67 up to 10% Caplin M et al. , NEJM 2014; Rinke A et al. , J Clin Oncol 2009; Yao et al. , NEJM 2011; Raymond et al. NEJM 2011; Strosberg J, et al. NEJM 2016; Yao JC, et al. Lancet 2016;

PREGUNTA…. ? SECUENCIA DE TRATAMIENTO ? ? SELECCIÓN DE PACIENTES ? ?

PREGUNTA…. ? SECUENCIA DE TRATAMIENTO ? ? SELECCIÓN DE PACIENTES ? ?

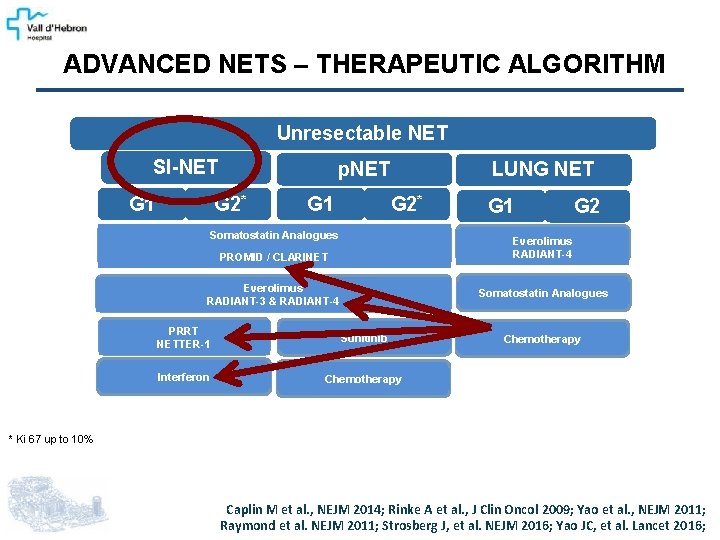
ADVANCED NETS – THERAPEUTIC ALGORITHM Unresectable NET SI-NET G 1 p. NET G 2* G 1 LUNG NET G 2* Somatostatin Analogues G 1 G 2 Everolimus RADIANT-4 PROMID / CLARINET Everolimus RADIANT-3 & RADIANT-4 Somatostatin Analogues PRRT NETTER-1 Sunitinib Interferon Chemotherapy * Ki 67 up to 10% Caplin M et al. , NEJM 2014; Rinke A et al. , J Clin Oncol 2009; Yao et al. , NEJM 2011; Raymond et al. NEJM 2011; Strosberg J, et al. NEJM 2016; Yao JC, et al. Lancet 2016;

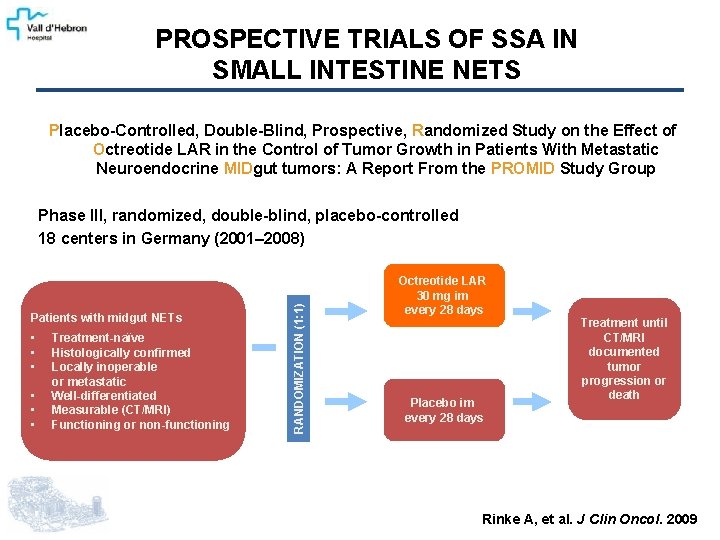
PROSPECTIVE TRIALS OF SSA IN SMALL INTESTINE NETS Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine MIDgut tumors: A Report From the PROMID Study Group Patients with midgut NETs • • • Treatment-naïve Histologically confirmed Locally inoperable or metastatic Well-differentiated Measurable (CT/MRI) Functioning or non-functioning RANDOMIZATION (1: 1) Phase III, randomized, double-blind, placebo-controlled 18 centers in Germany (2001– 2008) Octreotide LAR 30 mg im every 28 days Placebo im every 28 days Treatment until CT/MRI documented tumor progression or death Rinke A, et al. J Clin Oncol. 2009

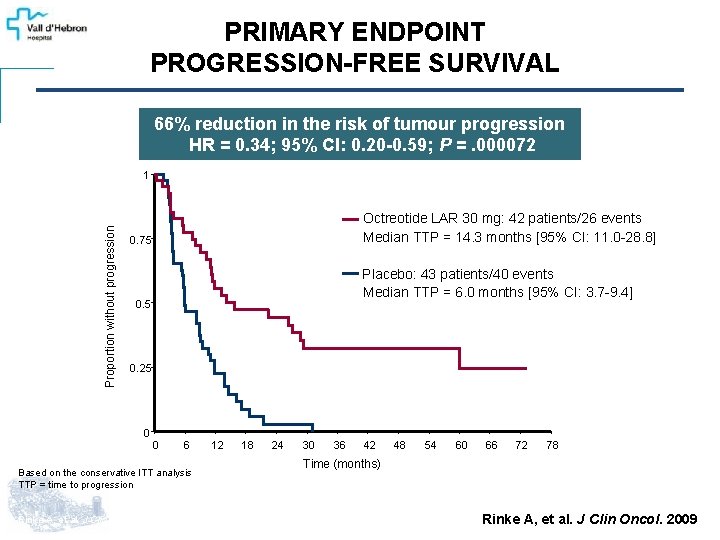
PRIMARY ENDPOINT PROGRESSION-FREE SURVIVAL 66% reduction in the risk of tumour progression HR = 0. 34; 95% CI: 0. 20 -0. 59; P =. 000072 Proportion without progression 1 Octreotide LAR 30 mg: 42 patients/26 events Median TTP = 14. 3 months [95% CI: 11. 0 -28. 8] 0. 75 Placebo: 43 patients/40 events Median TTP = 6. 0 months [95% CI: 3. 7 -9. 4] 0. 5 0. 25 0 0 6 12 18 Based on the conservative ITT analysis TTP = time to progression Rinke A, et al. J Clin Oncol. 2009; 27(28): 4656 -4663. 24 30 36 42 48 54 60 66 72 78 Time (months) Rinke A, et al. J Clin Oncol. 2009

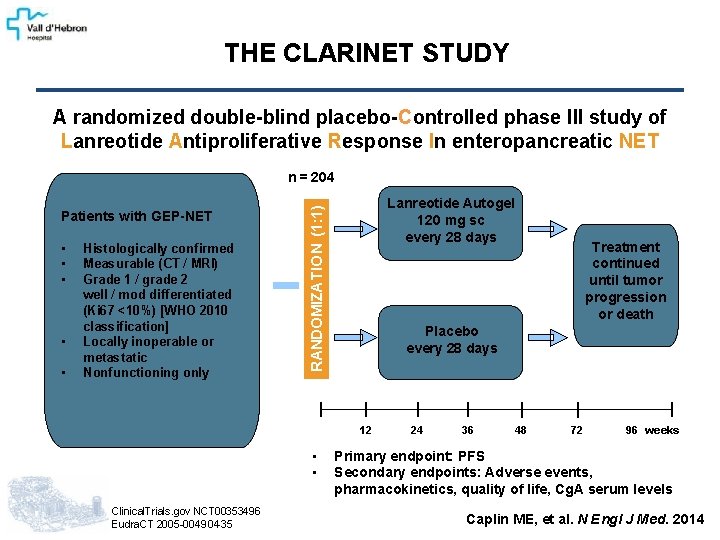
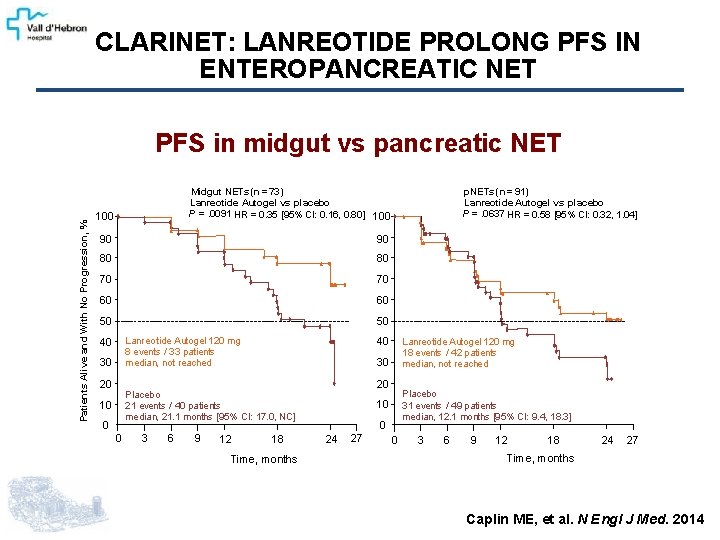
THE CLARINET STUDY A randomized double-blind placebo-Controlled phase III study of Lanreotide Antiproliferative Response In enteropancreatic NET n = 204 • • • Histologically confirmed Measurable (CT / MRI) Grade 1 / grade 2 well / mod differentiated (Ki 67 <10%) [WHO 2010 classification] Locally inoperable or metastatic Nonfunctioning only Lanreotide Autogel 120 mg sc every 28 days RANDOMIZATION (1: 1) Patients with GEP-NET Placebo every 28 days 12 • • Clinical. Trials. gov NCT 00353496 Eudra. CT 2005 -004904 -35 Treatment continued until tumor progression or death 24 36 48 72 96 weeks Primary endpoint: PFS Secondary endpoints: Adverse events, pharmacokinetics, quality of life, Cg. A serum levels Caplin ME, et al. N Engl J Med. 2014

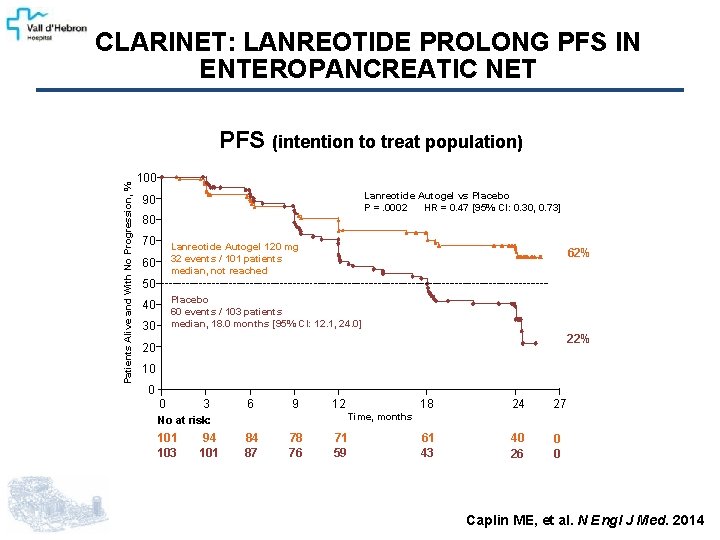
CLARINET: LANREOTIDE PROLONG PFS IN ENTEROPANCREATIC NET Patients Alive and With No Progression, % PFS (intention to treat population) 100 Lanreotide Autogel vs Placebo P =. 0002 HR = 0. 47 [95% CI: 0. 30, 0. 73] 90 80 70 Lanreotide Autogel 120 mg 32 events / 101 patients median, not reached 60 62% 50 Placebo 60 events / 103 patients median, 18. 0 months [95% CI: 12. 1, 24. 0] 40 30 22% 20 10 0 0 3 6 9 94 101 18 24 27 61 43 40 26 0 0 Time, months No at risk: 101 103 12 84 87 78 76 71 59 Caplin ME, et al. N Engl J Med. 2014

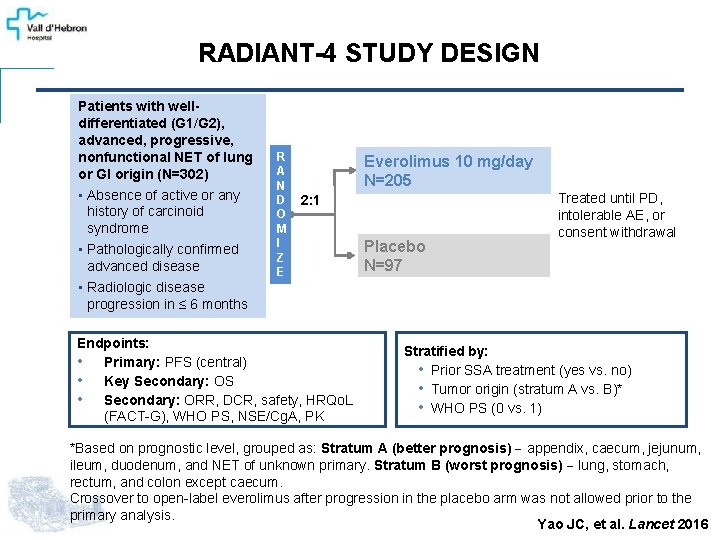
RADIANT-4 STUDY DESIGN Patients with welldifferentiated (G 1/G 2), advanced, progressive, nonfunctional NET of lung or GI origin (N=302) • Absence of active or any history of carcinoid syndrome • Pathologically confirmed advanced disease • Radiologic disease progression in ≤ 6 months R A N D O M I Z E Everolimus 10 mg/day N=205 2: 1 Endpoints: • Primary: PFS (central) • Key Secondary: OS • Secondary: ORR, DCR, safety, HRQo. L (FACT-G), WHO PS, NSE/Cg. A, PK Placebo N=97 Treated until PD, intolerable AE, or consent withdrawal Stratified by: • Prior SSA treatment (yes vs. no) • Tumor origin (stratum A vs. B)* • WHO PS (0 vs. 1) *Based on prognostic level, grouped as: Stratum A (better prognosis) appendix, caecum, jejunum, ileum, duodenum, and NET of unknown primary. Stratum B (worst prognosis) lung, stomach, rectum, and colon except caecum. Crossover to open-label everolimus after progression in the placebo arm was not allowed prior to the primary analysis. Yao JC, et al. Lancet 2016

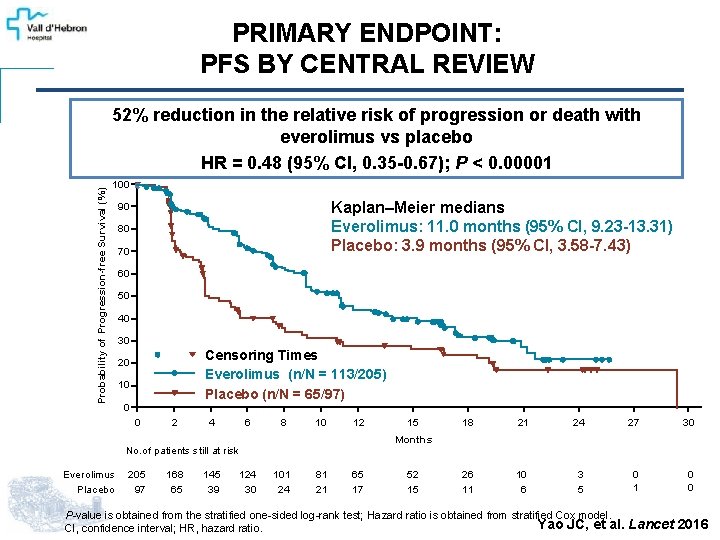
PRIMARY ENDPOINT: PFS BY CENTRAL REVIEW Probability of Progression-free Survival (%) 52% reduction in the relative risk of progression or death with everolimus vs placebo HR = 0. 48 (95% CI, 0. 35 -0. 67); P < 0. 00001 100 Kaplan–Meier medians Everolimus: 11. 0 months (95% CI, 9. 23 -13. 31) Placebo: 3. 9 months (95% CI, 3. 58 -7. 43) 90 80 70 60 50 40 30 Censoring Times Everolimus (n/N = 113/205) Placebo (n/N = 65/97) 20 10 0 0 2 4 6 8 10 12 205 97 168 65 145 39 124 30 18 21 24 27 30 26 11 10 6 3 5 0 1 0 0 Months No. of patients still at risk Everolimus Placebo 15 101 24 81 21 65 17 52 15 P-value is obtained from the stratified one-sided log-rank test; Hazard ratio is obtained from stratified Cox model. Yao JC, et al. CI, confidence interval; HR, hazard ratio. Lancet 2016

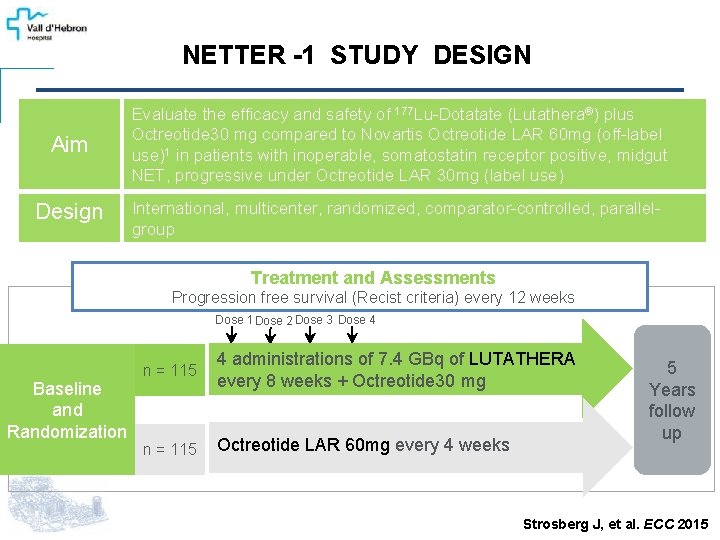
NETTER -1 STUDY DESIGN Aim Evaluate the efficacy and safety of 177 Lu-Dotatate (Lutathera®) plus Octreotide 30 mg compared to Novartis Octreotide LAR 60 mg (off-label use)1 in patients with inoperable, somatostatin receptor positive, midgut NET, progressive under Octreotide LAR 30 mg (label use) Design International, multicenter, randomized, comparator-controlled, parallelgroup Treatment and Assessments Progression free survival (Recist criteria) every 12 weeks Dose 1 Dose 2 Dose 3 Dose 4 Baseline and Randomization n = 115 4 administrations of 7. 4 GBq of LUTATHERA every 8 weeks + Octreotide 30 mg n = 115 Octreotide LAR 60 mg every 4 weeks 5 Years follow up Strosberg J, et al. ECC 2015

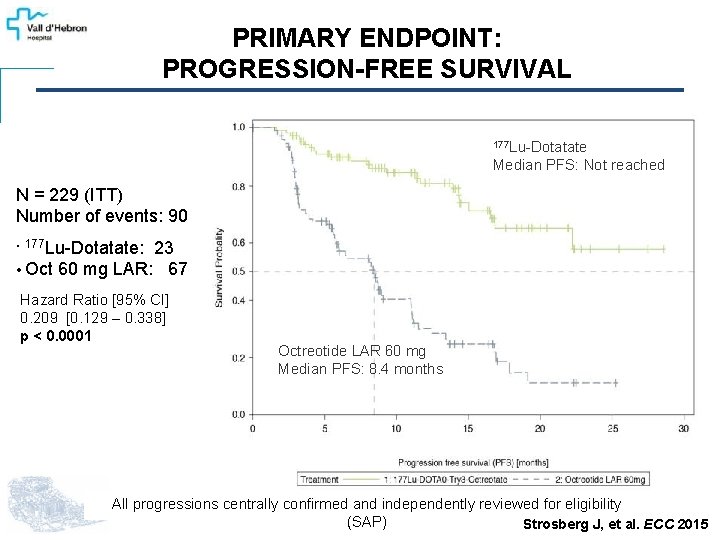
PRIMARY ENDPOINT: PROGRESSION-FREE SURVIVAL 177 Lu-Dotatate Median PFS: Not reached N = 229 (ITT) Number of events: 90 23 • Oct 60 mg LAR: 67 • 177 Lu-Dotatate: Hazard Ratio [95% CI] 0. 209 [0. 129 – 0. 338] p < 0. 0001 Octreotide LAR 60 mg Median PFS: 8. 4 months All progressions centrally confirmed and independently reviewed for eligibility (SAP) Strosberg J, et al. ECC 2015

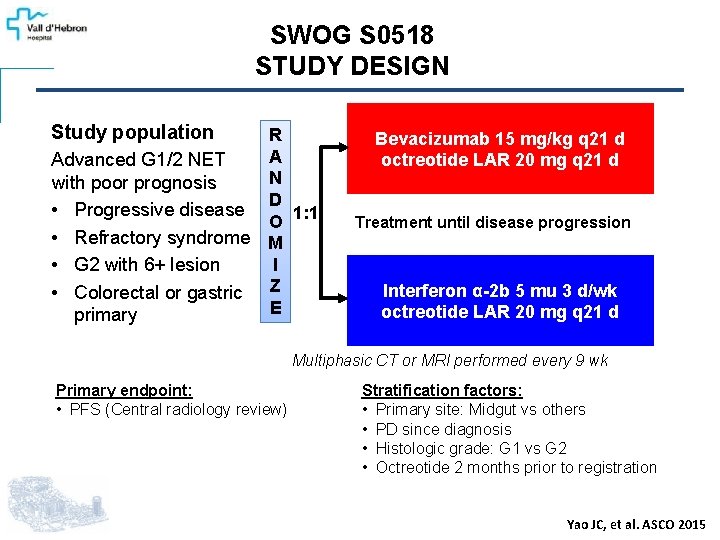
SWOG S 0518 STUDY DESIGN Study population R A Advanced G 1/2 NET N with poor prognosis D • Progressive disease O 1: 1 • Refractory syndrome M I • G 2 with 6+ lesion • Colorectal or gastric Z E primary Bevacizumab 15 mg/kg q 21 d octreotide LAR 20 mg q 21 d Treatment until disease progression Interferon α-2 b 5 mu 3 d/wk octreotide LAR 20 mg q 21 d Multiphasic CT or MRI performed every 9 wk Primary endpoint: • PFS (Central radiology review) Stratification factors: • Primary site: Midgut vs others • PD since diagnosis • Histologic grade: G 1 vs G 2 • Octreotide 2 months prior to registration Yao JC, et al. ASCO 2015

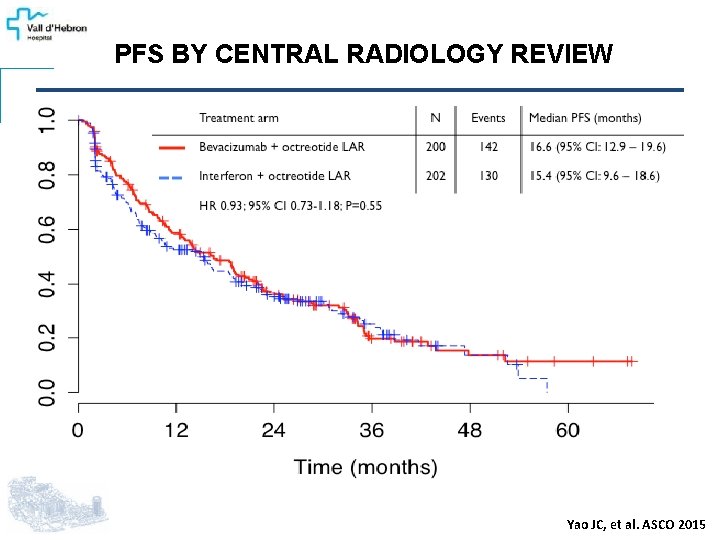
PFS BY CENTRAL RADIOLOGY REVIEW Yao JC, et al. ASCO 2015

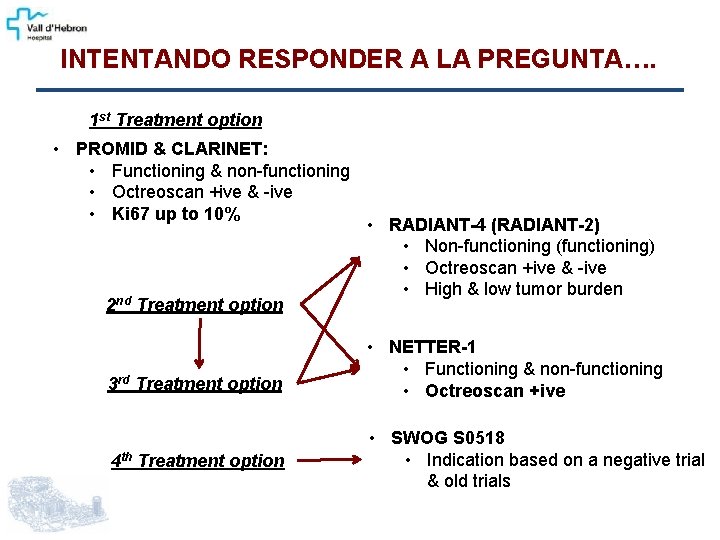
INTENTANDO RESPONDER A LA PREGUNTA…. 1 st Treatment option • PROMID & CLARINET: • Functioning & non-functioning • Octreoscan +ive & -ive • Ki 67 up to 10% 2 nd Treatment option 3 rd Treatment option 4 th Treatment option • RADIANT-4 (RADIANT-2) • Non-functioning (functioning) • Octreoscan +ive & -ive • High & low tumor burden • NETTER-1 • Functioning & non-functioning • Octreoscan +ive • SWOG S 0518 • Indication based on a negative trial & old trials

ADVANCED NETS – THERAPEUTIC ALGORITHM Unresectable NET SI-NET G 1 p. NET G 2* G 1 LUNG NET G 2* Somatostatin Analogues G 1 G 2 Everolimus RADIANT-4 PROMID / CLARINET Everolimus RADIANT-3 & RADIANT-4 Somatostatin Analogues PRRT NETTER-1 Sunitinib Interferon Chemotherapy * Ki 67 up to 10% Caplin M et al. , NEJM 2014; Rinke A et al. , J Clin Oncol 2009; Yao et al. , NEJM 2011; Raymond et al. NEJM 2011; Strosberg J, et al. NEJM 2016; Yao JC, et al. Lancet 2016;

CLARINET: LANREOTIDE PROLONG PFS IN ENTEROPANCREATIC NET Patients Alive and With No Progression, % PFS in midgut vs pancreatic NET 100 Midgut NETs (n =73) Lanreotide Autogel vs placebo P =. 0091 HR = 0. 35 [95% CI: 0. 16, 0. 80] 100 90 90 80 80 70 70 60 60 50 50 Lanreotide Autogel 120 mg 8 events / 33 patients median, not reached 40 30 20 40 0 0 3 6 9 12 Lanreotide Autogel 120 mg 18 events / 42 patients median, not reached 30 20 Placebo 21 events / 40 patients median, 21. 1 months [95% CI: 17. 0, NC] 10 p. NETs (n = 91) Lanreotide Autogel vs placebo P =. 0637 HR = 0. 58 [95% CI: 0. 32, 1. 04] 18 Time, months Placebo 31 events / 49 patients median, 12. 1 months [95% CI: 9. 4, 18. 3] 10 0 24 27 0 3 6 9 12 18 24 27 Time, months Caplin ME, et al. N Engl J Med. 2014

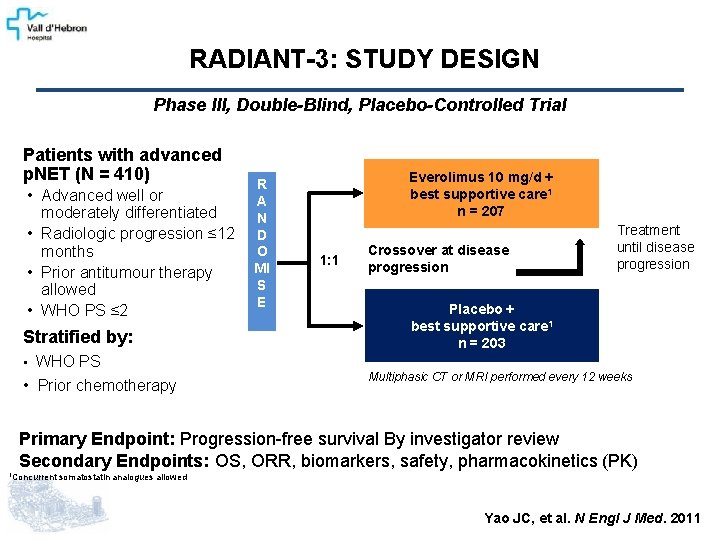
RADIANT-3: STUDY DESIGN Phase III, Double-Blind, Placebo-Controlled Trial Patients with advanced p. NET (N = 410) • Advanced well or moderately differentiated • Radiologic progression ≤ 12 months • Prior antitumour therapy allowed • WHO PS ≤ 2 Stratified by: • WHO PS • Prior chemotherapy R A N D O MI S E Everolimus 10 mg/d + best supportive care 1 n = 207 1: 1 Crossover at disease progression Treatment until disease progression Placebo + best supportive care 1 n = 203 Multiphasic CT or MRI performed every 12 weeks Primary Endpoint: Progression-free survival By investigator review Secondary Endpoints: OS, ORR, biomarkers, safety, pharmacokinetics (PK) 1 Concurrent somatostatin analogues allowed Yao JC, et al. N Engl J Med. 2011

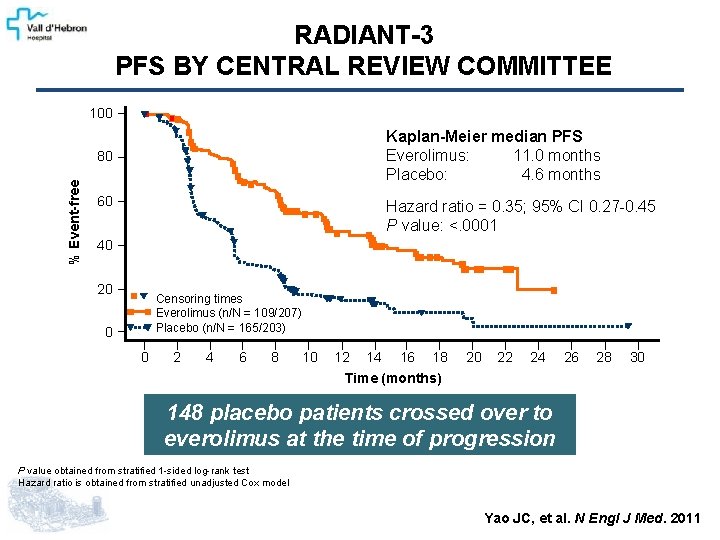
RADIANT-3 PFS BY CENTRAL REVIEW COMMITTEE 100 Kaplan-Meier median PFS Everolimus: 11. 0 months Placebo: 4. 6 months % Event-free 80 60 Hazard ratio = 0. 35; 95% CI 0. 27 -0. 45 P value: <. 0001 40 20 Censoring times Everolimus (n/N = 109/207) Placebo (n/N = 165/203) 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Time (months) 148 placebo patients crossed over to everolimus at the time of progression P value obtained from stratified 1 -sided log-rank test Hazard ratio is obtained from stratified unadjusted Cox model Yao JC, et al. N Engl J Med. 2011

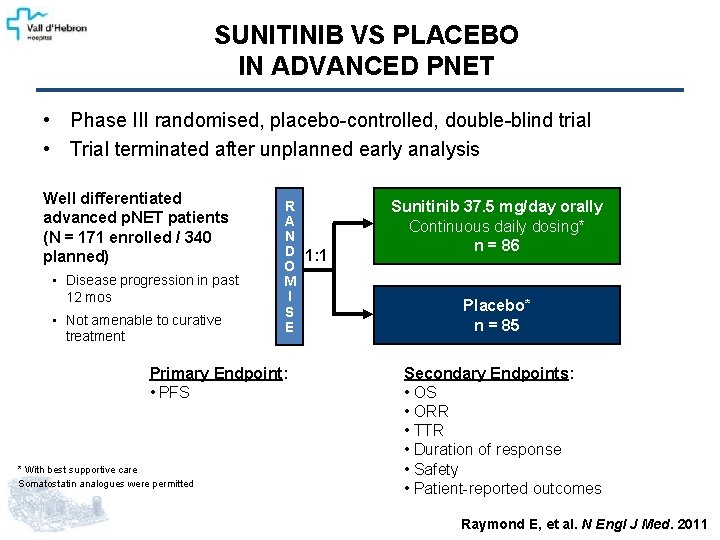
SUNITINIB VS PLACEBO IN ADVANCED PNET • Phase III randomised, placebo-controlled, double-blind trial • Trial terminated after unplanned early analysis Well differentiated advanced p. NET patients (N = 171 enrolled / 340 planned) • Disease progression in past 12 mos • Not amenable to curative treatment R A N D 1: 1 O M I S E Primary Endpoint: • PFS * With best supportive care Somatostatin analogues were permitted Sunitinib 37. 5 mg/day orally Continuous daily dosing* n = 86 Placebo* n = 85 Secondary Endpoints: • OS • ORR • TTR • Duration of response • Safety • Patient-reported outcomes Raymond E, et al. N Engl J Med. 2011

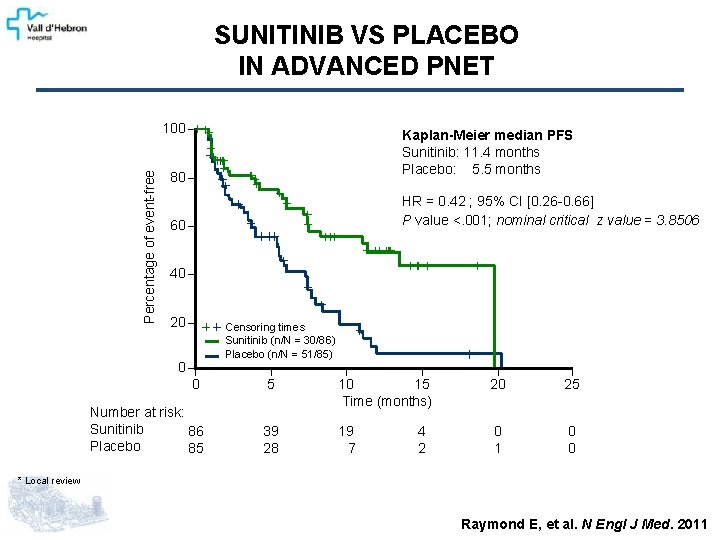
SUNITINIB VS PLACEBO IN ADVANCED PNET Percentage of event-free 100 Kaplan-Meier median PFS Sunitinib: 11. 4 months Placebo: 5. 5 months 80 HR = 0. 42 ; 95% CI [0. 26 -0. 66] P value <. 001; nominal critical z value = 3. 8506 60 40 20 Censoring times Sunitinib (n/N = 30/86) Placebo (n/N = 51/85) 0 0 5 20 25 Number at risk: Sunitinib 86 Placebo 85 10 15 Time (months) 39 28 19 7 0 1 0 0 4 2 * Local review Raymond E, et al. N Engl J Med. 2011

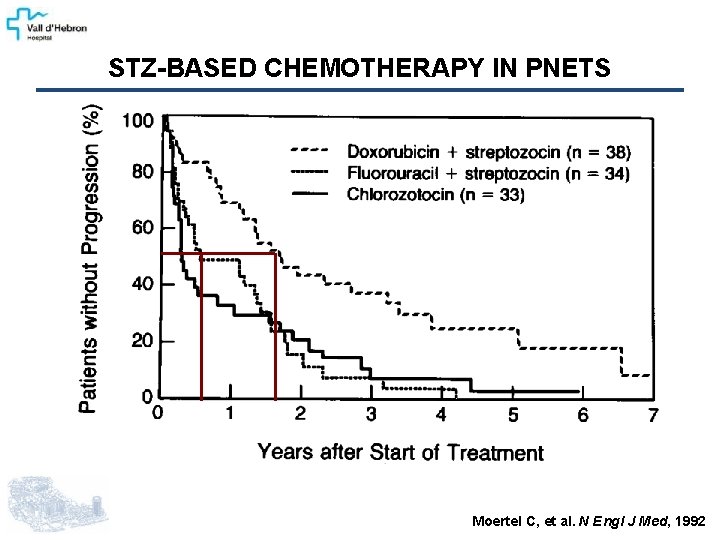
STZ-BASED CHEMOTHERAPY IN PNETS Moertel C, et al. N Engl J Med, 1992

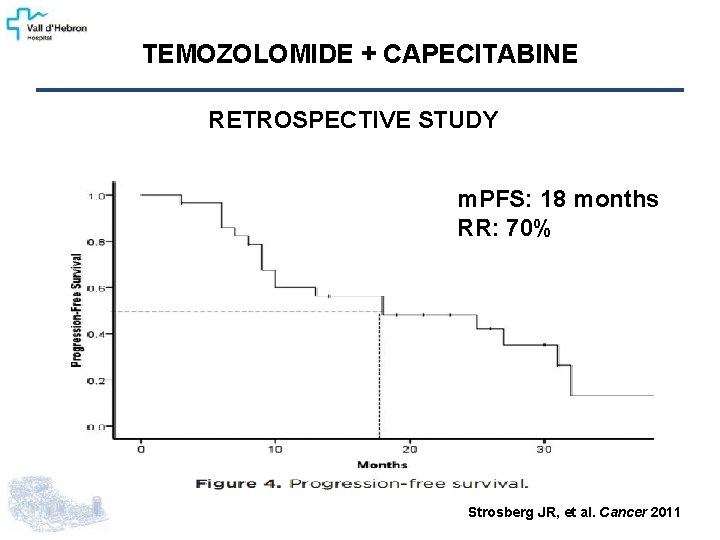
TEMOZOLOMIDE + CAPECITABINE RETROSPECTIVE STUDY m. PFS: 18 months RR: 70% Strosberg JR, et al. Cancer 2011

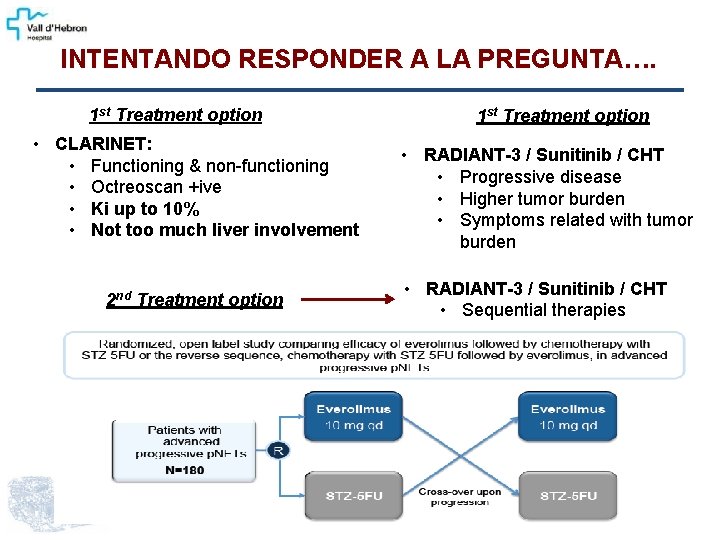
INTENTANDO RESPONDER A LA PREGUNTA…. 1 st Treatment option • CLARINET: • Functioning & non-functioning • Octreoscan +ive • Ki up to 10% • Not too much liver involvement 2 nd Treatment option 1 st Treatment option • RADIANT-3 / Sunitinib / CHT • Progressive disease • Higher tumor burden • Symptoms related with tumor burden • RADIANT-3 / Sunitinib / CHT • Sequential therapies

ADVANCED NETS – THERAPEUTIC ALGORITHM Unresectable NET SI-NET G 1 p. NET G 2* G 1 LUNG NET G 2* Somatostatin Analogues G 1 G 2 Everolimus RADIANT-4 PROMID / CLARINET Everolimus RADIANT-3 & RADIANT-4 Somatostatin Analogues PRRT NETTER-1 Sunitinib Interferon Chemotherapy * Ki 67 up to 10% Caplin M et al. , NEJM 2014; Rinke A et al. , J Clin Oncol 2009; Yao et al. , NEJM 2011; Raymond et al. NEJM 2011; Strosberg J, et al. NEJM 2016; Yao JC, et al. Lancet 2016;

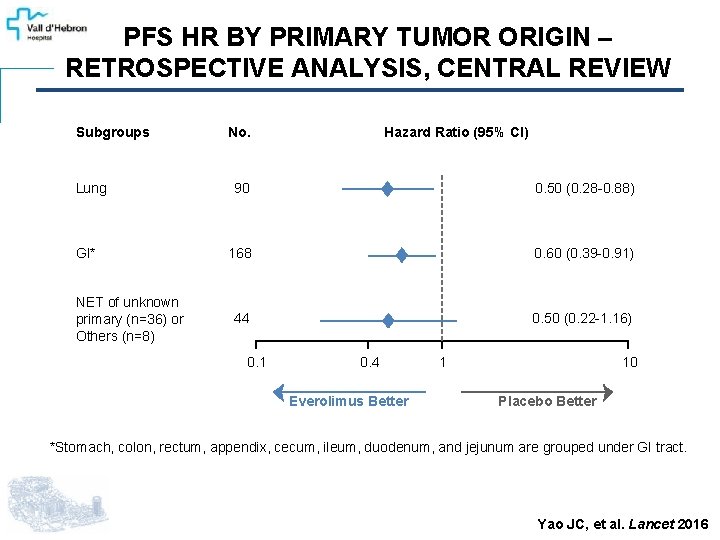
PFS HR BY PRIMARY TUMOR ORIGIN – RETROSPECTIVE ANALYSIS, CENTRAL REVIEW Subgroups Lung GI* NET of unknown primary (n=36) or Others (n=8) No. Hazard Ratio (95% CI) 90 0. 50 (0. 28 -0. 88) 168 0. 60 (0. 39 -0. 91) 44 0. 50 (0. 22 -1. 16) 0. 1 0. 4 Everolimus Better 1 10 Placebo Better *Stomach, colon, rectum, appendix, cecum, ileum, duodenum, and jejunum are grouped under GI tract. Yao JC, et al. Lancet 2016

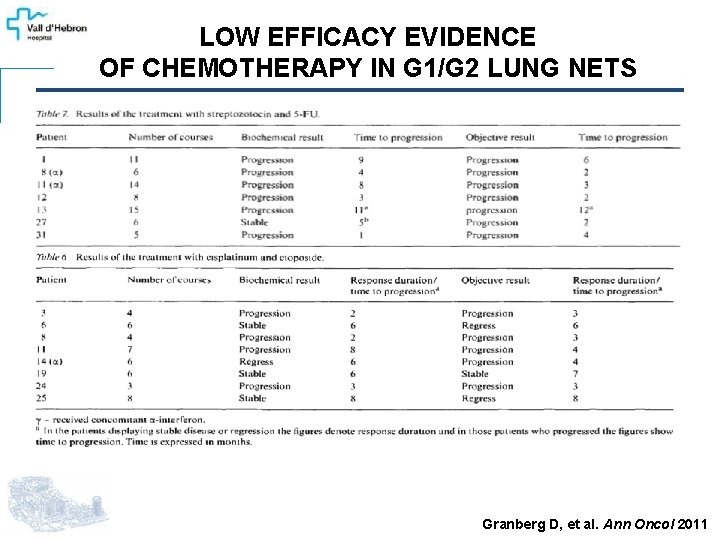
LOW EFFICACY EVIDENCE OF CHEMOTHERAPY IN G 1/G 2 LUNG NETS Granberg D, et al. Ann Oncol 2011

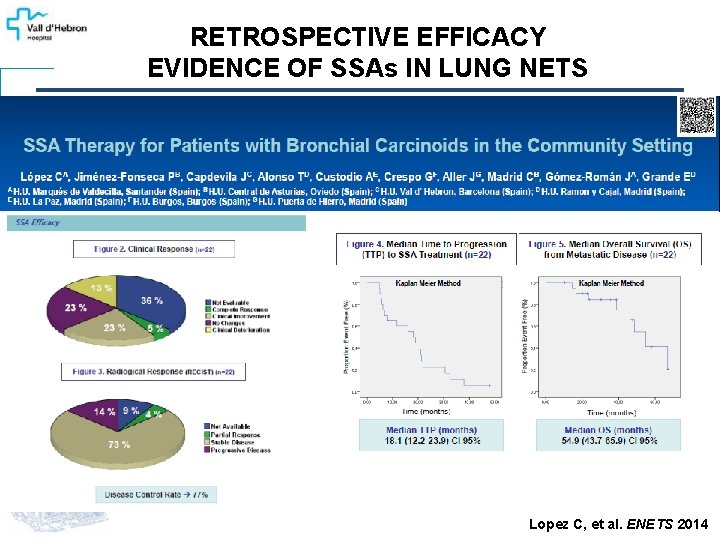
RETROSPECTIVE EFFICACY EVIDENCE OF SSAs IN LUNG NETS Lopez C, et al. ENETS 2014

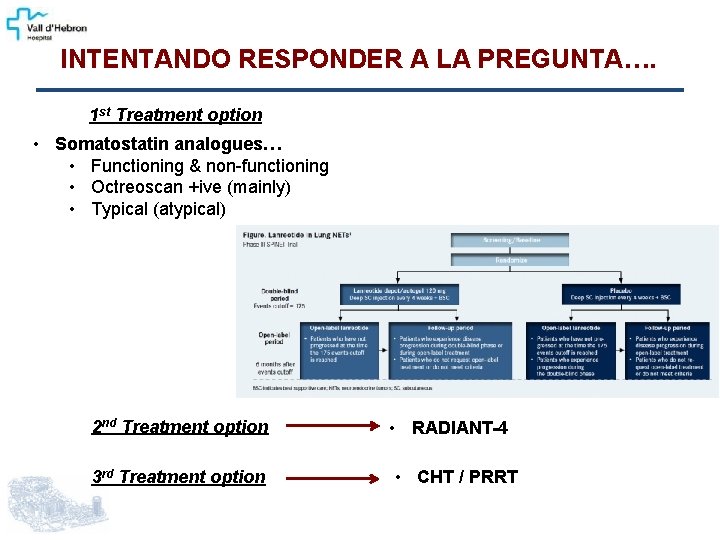
INTENTANDO RESPONDER A LA PREGUNTA…. 1 st Treatment option • Somatostatin analogues… • Functioning & non-functioning • Octreoscan +ive (mainly) • Typical (atypical) 2 nd Treatment option 3 rd Treatment option • RADIANT-4 • CHT / PRRT

PREGUNTA…. ? SECUENCIA DE TRATAMIENTO ? ? SELECCIÓN DE PACIENTES ? ?

INTENTANDO RESPONDER A LA PREGUNTA…. • CLINIC & PATHOLOGICAL FACTORS • • • Grade / Differentiation Tumor burden Tumor origin SRS uptake Hormone release Progression status • MOLECULAR BIOMARKERS 2017/8/9…

MUCHAS GRACIAS POR VUESTRA ATENCIÓN jacapdevila@vhebron. net jcapdevila@onco. cat
 Diploma dea
Diploma dea Instituto de estudios avanzados siglo xxi
Instituto de estudios avanzados siglo xxi Centro de estudios avanzados de blanes
Centro de estudios avanzados de blanes Elaboración de documentos digitales avanzados
Elaboración de documentos digitales avanzados Conceptos de rol avanzados
Conceptos de rol avanzados Diploma de estudios avanzados dea
Diploma de estudios avanzados dea Secuencias reguladoras
Secuencias reguladoras Ejercicios de secuenciación
Ejercicios de secuenciación Secuencia textual argumentativa
Secuencia textual argumentativa El cielo protector en libre mente de fernando savater
El cielo protector en libre mente de fernando savater Secuencia textual ejemplos
Secuencia textual ejemplos Diagrama despliegue
Diagrama despliegue Blast alineamiento de secuencias
Blast alineamiento de secuencias Tengo muy presente la fisonomía del clérigo
Tengo muy presente la fisonomía del clérigo Jean michel adam secuencias textuales
Jean michel adam secuencias textuales Ejercicios de secuencias textuales
Ejercicios de secuencias textuales Acción educativa
Acción educativa Secuencia incompleta
Secuencia incompleta Esquemas textuales
Esquemas textuales Secuencias alfoides
Secuencias alfoides Secuencia narrativa ejercicios
Secuencia narrativa ejercicios Situacion inicial conflicto y desenlace
Situacion inicial conflicto y desenlace Secuencia formativa
Secuencia formativa Grupo funcional carbonilo
Grupo funcional carbonilo Gradient
Gradient Muscle alineamiento
Muscle alineamiento Forma discursiva narrativa ejemplos
Forma discursiva narrativa ejemplos Proso
Proso Foco tricuspideo
Foco tricuspideo Púrpura de schamberg causas
Púrpura de schamberg causas Que entendemos por lenguaje algebraico
Que entendemos por lenguaje algebraico Neumomediastino tratamiento
Neumomediastino tratamiento Diverticulitis tratamiento farmacologico
Diverticulitis tratamiento farmacologico Timoma benigno tratamiento
Timoma benigno tratamiento Intrusión dental
Intrusión dental