Transposable elements Transposons TEs mobile genetic elements sequences

Transposable elements

Transposons, TEs = mobile genetic elements - sequences of DNA that can move around to different positions within the genome of a single cell (transposition), - cause mutations and can induce chromosomal rearrang. identified in Prokaryotes and all Eukaryotes: (with exception of parasitic Plasmodium falciparum) animals 3 -45%, fungi 2 -20%, plants 10 -85% in plants: - thousands of families, forming majority of repetitive DNA
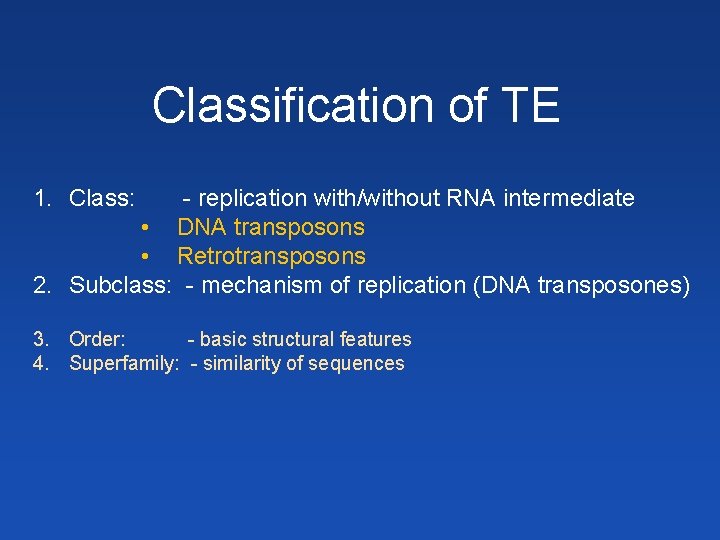
Classification of TE 1. Class: - replication with/without RNA intermediate • DNA transposons • Retrotransposons 2. Subclass: - mechanism of replication (DNA transposones) 3. Order: - basic structural features 4. Superfamily: - similarity of sequences
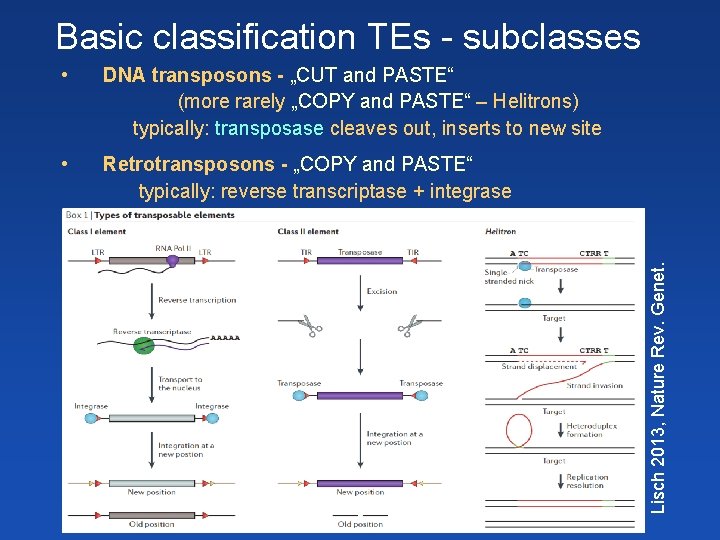
• DNA transposons - „CUT and PASTE“ (more rarely „COPY and PASTE“ – Helitrons) typically: transposase cleaves out, inserts to new site • Retrotransposons - „COPY and PASTE“ typically: reverse transcriptase + integrase Lisch 2013, Nature Rev. Genet. Basic classification TEs - subclasses
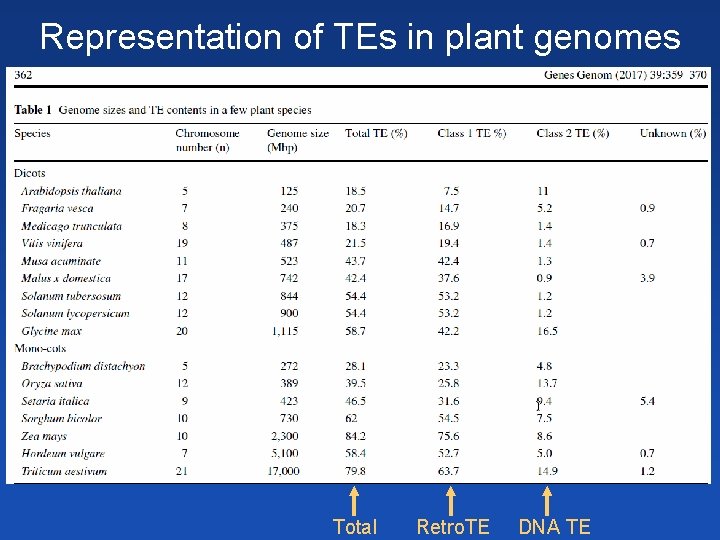
Representation of TEs in plant genomes Total Retro. TE DNA TE

Class II: DNA transposons

DNA transposons - subclass I transposition: break, religation - terminal inverted repeats recognized by transposase - duplication of short seq. (2 -8 bp) = footprint after transposition - clustering in genome, hundreds of copies - Ac, Spm, Mu (maize), Tam (Antirrhinum), Tph. I (petunia), Tag. I (Arabidopsis) - auto-/nonautonomous pairs: Ac/Ds, Spm/d. Spm - Stowaway, Tourist >10 000 copies
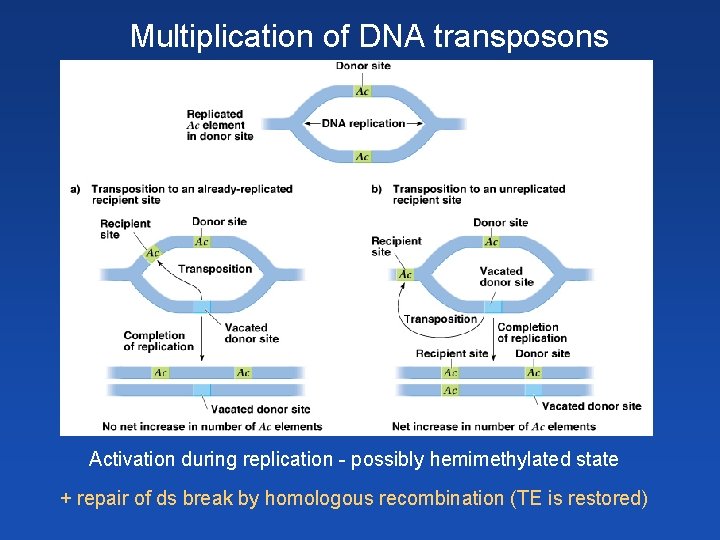
Multiplication of DNA transposons Activation during replication - possibly hemimethylated state + repair of ds break by homologous recombination (TE is restored)
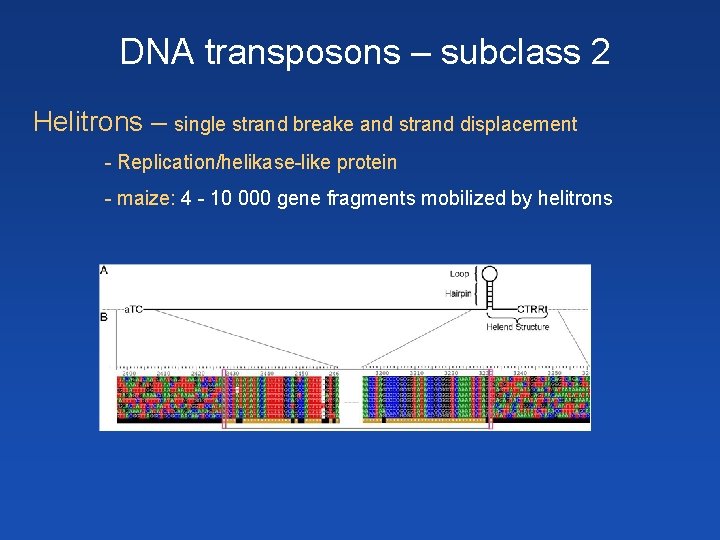
DNA transposons – subclass 2 Helitrons – single strand breake and strand displacement - Replication/helikase-like protein - maize: 4 - 10 000 gene fragments mobilized by helitrons
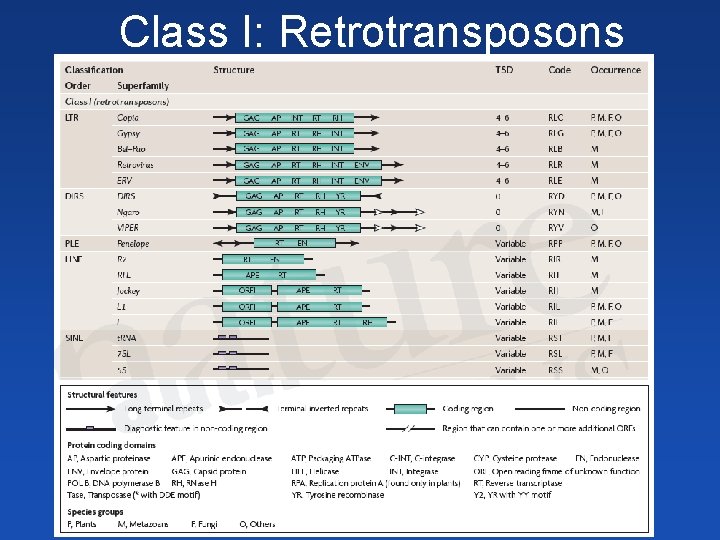
Class I: Retrotransposons
Retrotransposons - replication through RNA intermediate (multiple offspring) - related to retroviruses - millions of copies - huge portions of genome (up to 40 -80 % of genome size) - element size 1 -13 kbp Order LTR – most important in plants - LTR (long terminal repeat) - promotor, terminator, direct repeat - dubling of short target sequence - gag (nucleocapsid), - pol (protease, reverse transcriptase-RNase H, integrase)
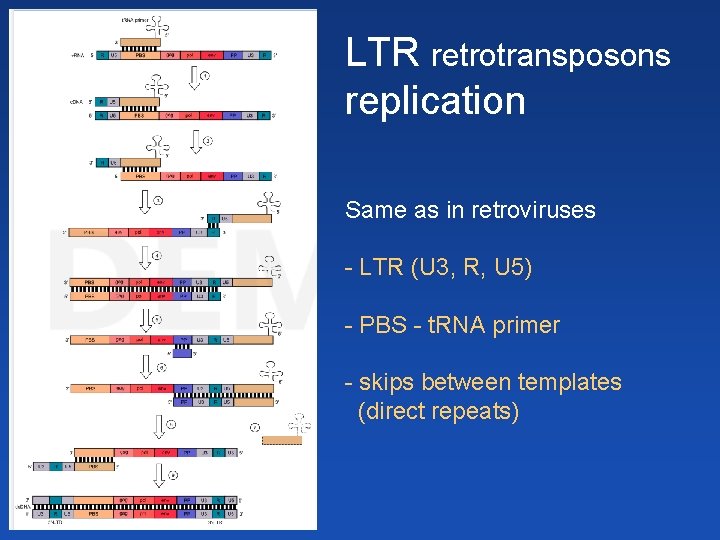
LTR retrotransposons replication Same as in retroviruses - LTR (U 3, R, U 5) - PBS - t. RNA primer - skips between templates (direct repeats)
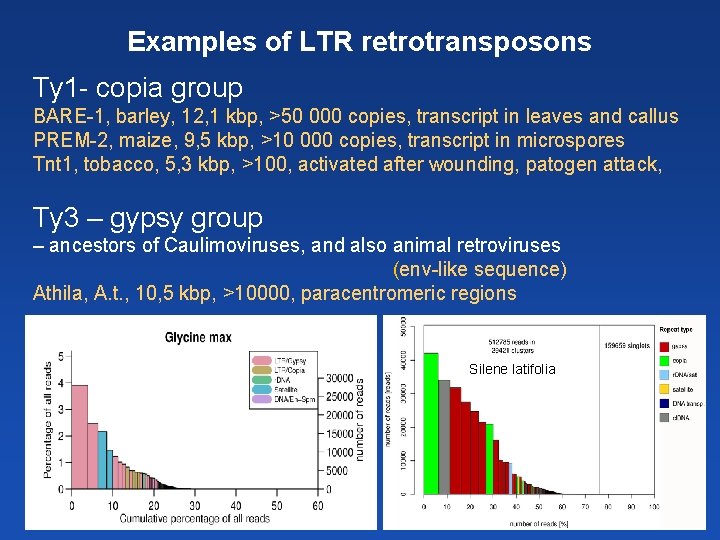
Examples of LTR retrotransposons Ty 1 - copia group BARE-1, barley, 12, 1 kbp, >50 000 copies, transcript in leaves and callus PREM-2, maize, 9, 5 kbp, >10 000 copies, transcript in microspores Tnt 1, tobacco, 5, 3 kbp, >100, activated after wounding, patogen attack, Ty 3 – gypsy group – ancestors of Caulimoviruses, and also animal retroviruses (env-like sequence) Athila, A. t. , 10, 5 kbp, >10000, paracentromeric regions Silene latifolia
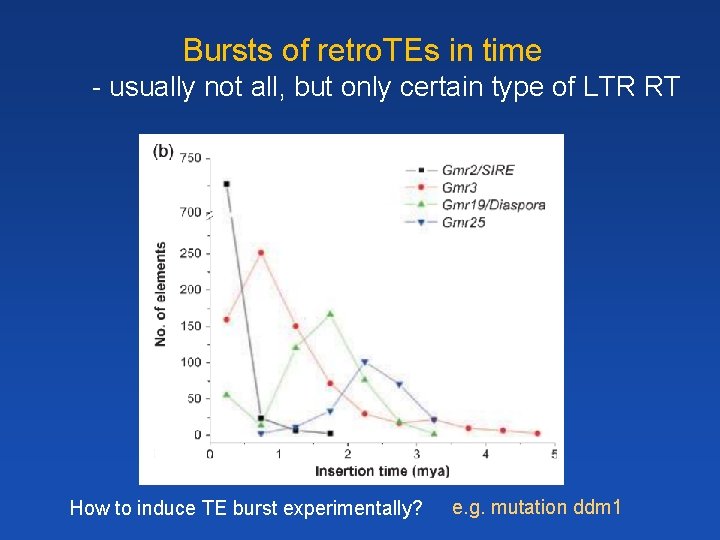
Bursts of retro. TEs in time - usually not all, but only certain type of LTR RT How to induce TE burst experimentally? e. g. mutation ddm 1

Retrotransposons without LTR LINE (long interspersed nuclear elements) SINE (short interspersed nuclear elements) LINE APE – endonuklease, RH – RNase H LINE - phylogenetically most original, ancestors of LTR - 5´region – promoter; 3´ region - terminator Cin 4, maize, 1 -6, 8 kbp, 50 -100, various truncated forms SINE - non-autonomous – use RT of other elements - derived from products of RNA polymerase III (t. RNA, 7 SLRNA, (r. RNA)) - < 500 nt
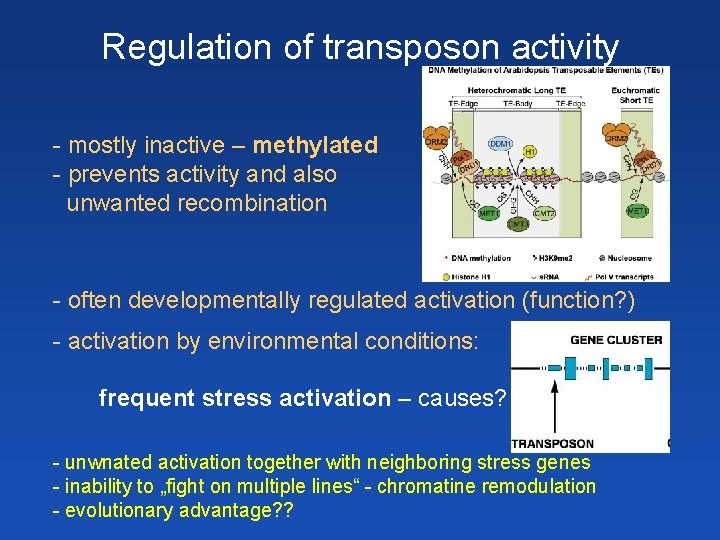
Regulation of transposon activity - mostly inactive – methylated - prevents activity and also unwanted recombination - often developmentally regulated activation (function? ) - activation by environmental conditions: frequent stress activation – causes? - unwnated activation together with neighboring stress genes - inability to „fight on multiple lines“ - chromatine remodulation - evolutionary advantage? ?
Role of TE in evolution causing mutations = „composting“ of unnecessary genetic information – MECHANISM? = increasing variability = generation of new sequences improving fitness by random mutation – low probability, but possible TE mutations: - gene knock-outs - gene multiplications - modulation of expression – activation x repression – developmentally- or stress-induced - new gene evolution – fusion genes (MULEs, Helitrons) - genome structure changes
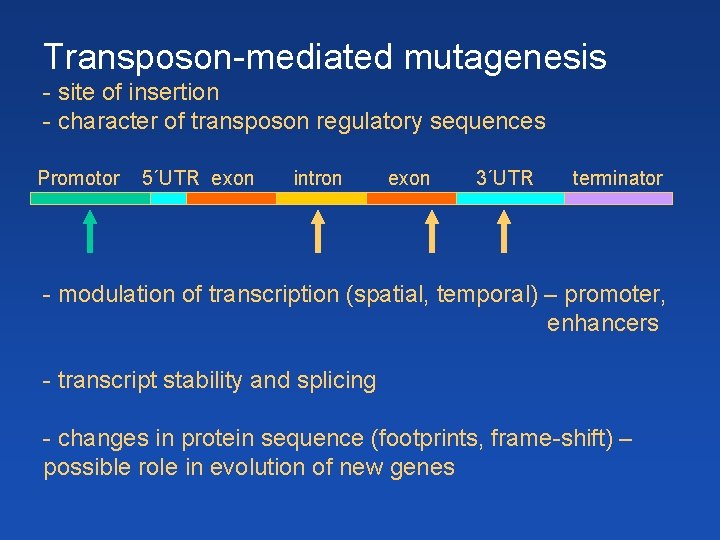
Transposon-mediated mutagenesis - site of insertion - character of transposon regulatory sequences Promotor 5´UTR exon intron exon 3´UTR terminator - modulation of transcription (spatial, temporal) – promoter, enhancers - transcript stability and splicing - changes in protein sequence (footprints, frame-shift) – possible role in evolution of new genes
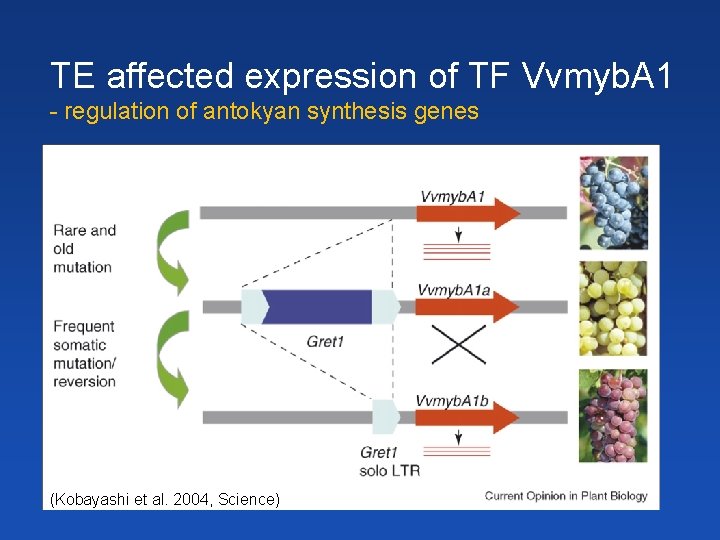
TE affected expression of TF Vvmyb. A 1 - regulation of antokyan synthesis genes (Kobayashi et al. 2004, Science)
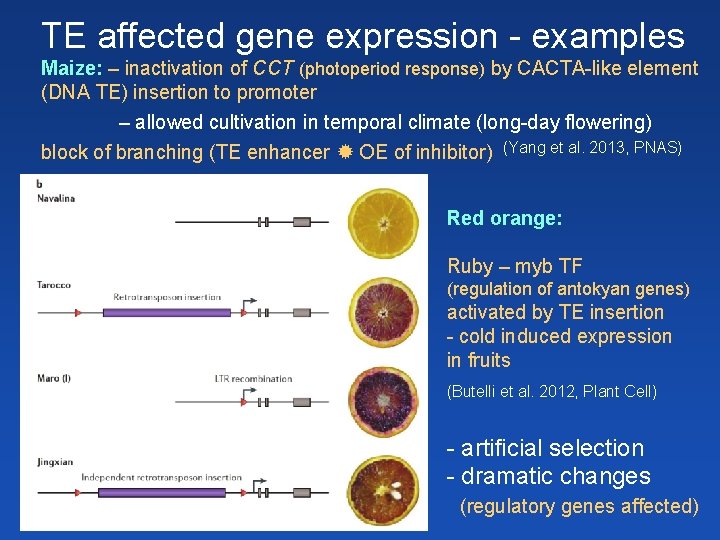
TE affected gene expression - examples Maize: – inactivation of CCT (photoperiod response) by CACTA-like element (DNA TE) insertion to promoter – allowed cultivation in temporal climate (long-day flowering) block of branching (TE enhancer OE of inhibitor) (Yang et al. 2013, PNAS) Red orange: Ruby – myb TF (regulation of antokyan genes) activated by TE insertion - cold induced expression in fruits (Butelli et al. 2012, Plant Cell) - artificial selection - dramatic changes (regulatory genes affected)
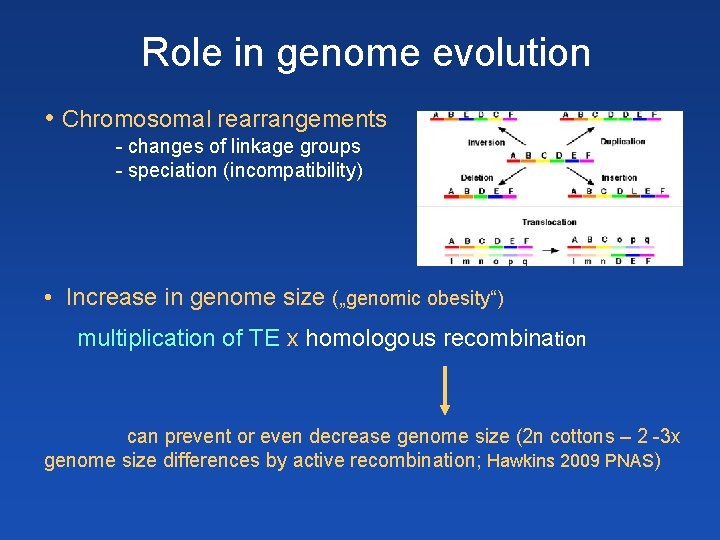
Role in genome evolution • Chromosomal rearrangements - changes of linkage groups - speciation (incompatibility) • Increase in genome size („genomic obesity“) multiplication of TE x homologous recombination can prevent or even decrease genome size (2 n cottons – 2 -3 x genome size differences by active recombination; Hawkins 2009 PNAS)
Classification of TE - based on self-sufficiency • Autonomous elements – encode genes necessary for transposition/ replication • Non-autonomous – derivatives of autonomous elements – lost of genes for transposition/replication – keep sequences necessary for transposition (can be mobilized by related autonomous elements!)
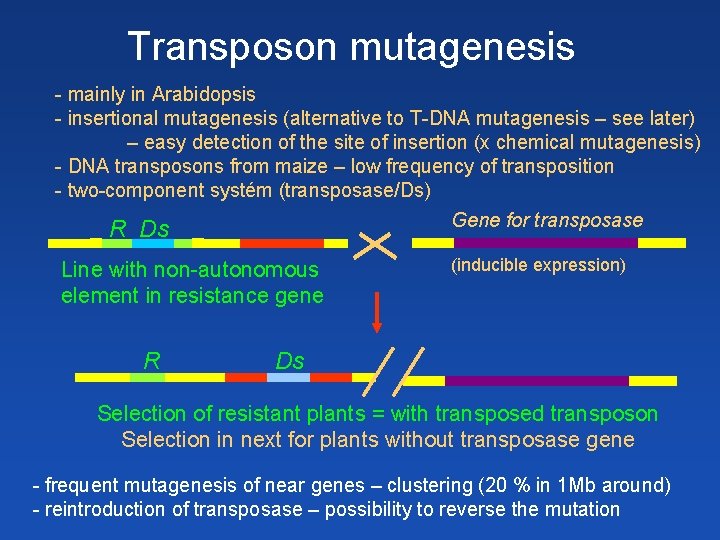
Transposon mutagenesis - mainly in Arabidopsis - insertional mutagenesis (alternative to T-DNA mutagenesis – see later) – easy detection of the site of insertion (x chemical mutagenesis) - DNA transposons from maize – low frequency of transposition - two-component systém (transposase/Ds) Gene for transposase R Ds Line with non-autonomous element in resistance gene R (inducible expression) Ds Selection of resistant plants = with transposed transposon Selection in next for plants without transposase gene - frequent mutagenesis of near genes – clustering (20 % in 1 Mb around) - reintroduction of transposase – possibility to reverse the mutation

Discovery of transposons Barbara Mc. Clintock (1902 -1992) Nobel prize in Physiology and Medicine 1983 Mobile genetic elements in maize 1940 -1950
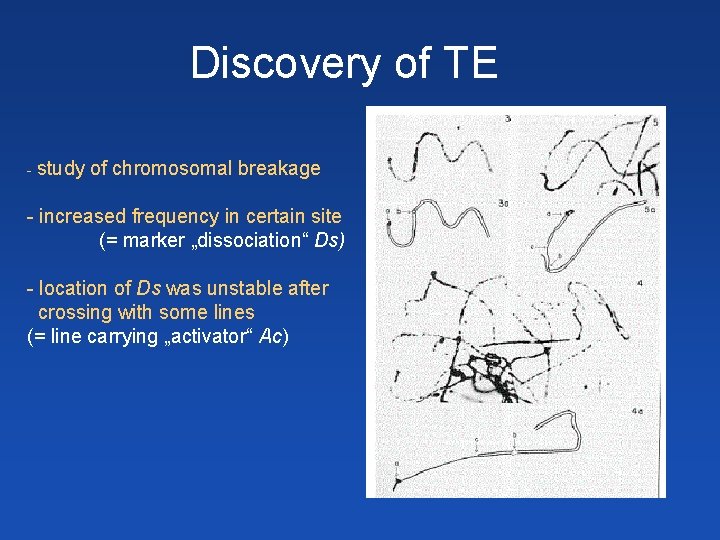
Discovery of TE - study of chromosomal breakage - increased frequency in certain site (= marker „dissociation“ Ds) - location of Ds was unstable after crossing with some lines (= line carrying „activator“ Ac)
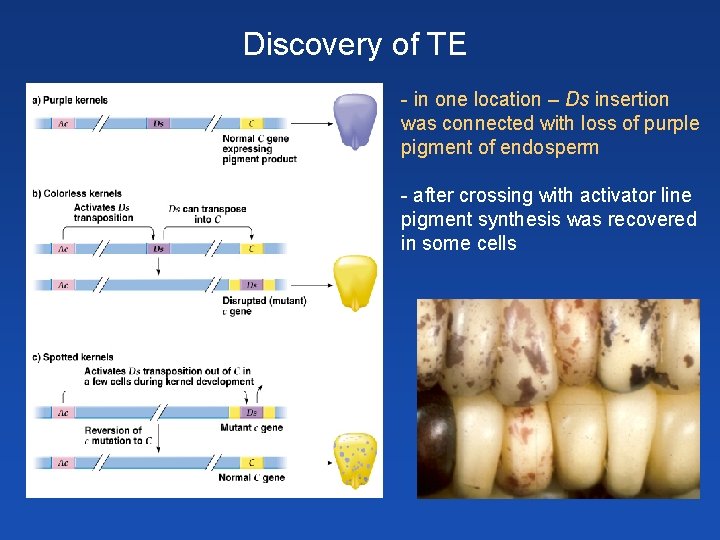
Discovery of TE - in one location – Ds insertion was connected with loss of purple pigment of endosperm - after crossing with activator line pigment synthesis was recovered in some cells
![Barbara Mc. Clintock (1902 -1992) 1951: formulated basic context of epigenetics: "[T]he progeny of Barbara Mc. Clintock (1902 -1992) 1951: formulated basic context of epigenetics: "[T]he progeny of](http://slidetodoc.com/presentation_image_h/ea16b8ed826f19b7b1407a2b12248912/image-27.jpg)
Barbara Mc. Clintock (1902 -1992) 1951: formulated basic context of epigenetics: "[T]he progeny of two (such) sister cells are not alike with respect to the types of gene alteration that will occur. Differential mitoses also produce the alterations that allow particular genes to be reactive. Other genes, although present, may remain inactive. This inactivity or suppression is considered to occur because the genes are ‘covered' by other nongenic chromatin materials. Gene activity may be possible only when a physical change in this covering material allows the reactive components of the gene to be ‘exposed' and thus capable of functioning. "
- Slides: 27