Transport molecules across plasma membrane 1 st Microtechnique

Transport molecules across plasma membrane 1 st Microtechnique Lab
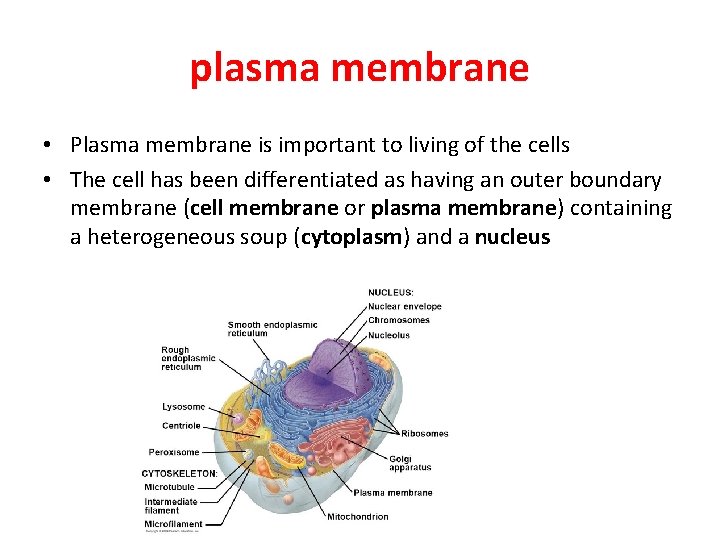
plasma membrane • Plasma membrane is important to living of the cells • The cell has been differentiated as having an outer boundary membrane (cell membrane or plasma membrane) containing a heterogeneous soup (cytoplasm) and a nucleus

• The plasma membrane, also known as the cell membrane defines as the boundary of the cell, it regulates the movement of materials into and out of the cell and facilitates electrical signaling between cells. • Other membranes define as the boundaries of organelles and provide a matrix upon which complex chemical reactions can occur.
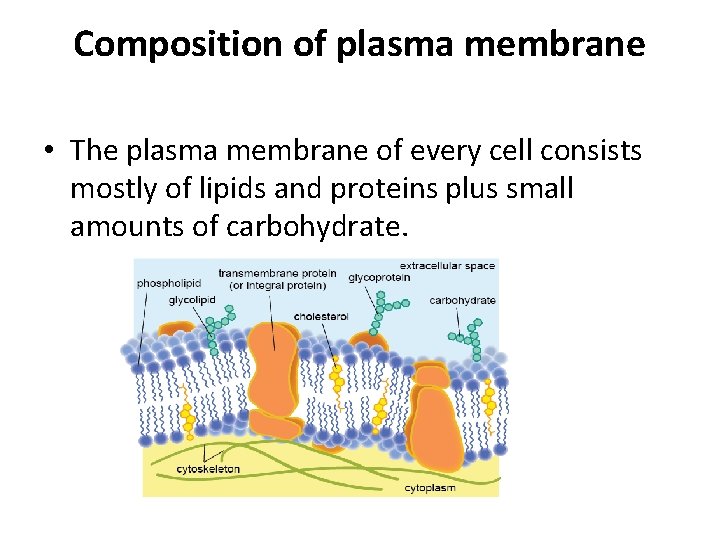
Composition of plasma membrane • The plasma membrane of every cell consists mostly of lipids and proteins plus small amounts of carbohydrate.
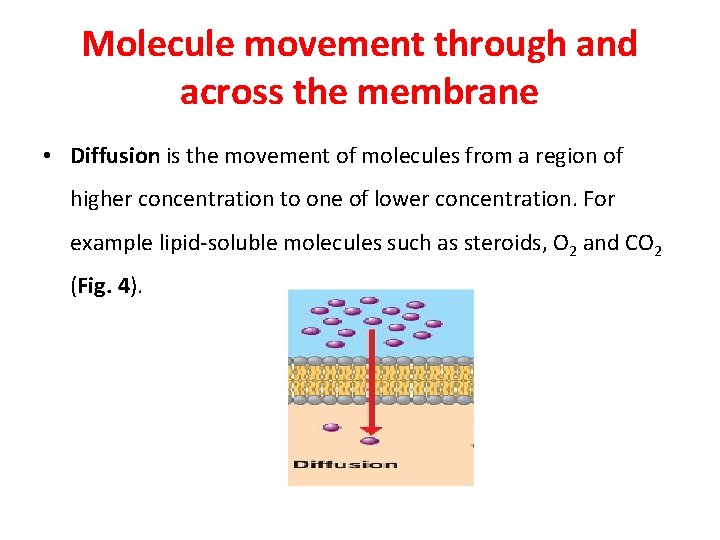
Molecule movement through and across the membrane • Diffusion is the movement of molecules from a region of higher concentration to one of lower concentration. For example lipid-soluble molecules such as steroids, O 2 and CO 2 (Fig. 4).
• Osmosis is the movement of water from a region of higher concentration water to one of lower concentration or from low concentration solute to higher concentration. Osmosis often occurs across a membrane that is semipermeable. A semipermeable membrane lets only certain molecules pass through while keeping other molecules out. Osmosis is really a type of diffusion involving only water molecules.
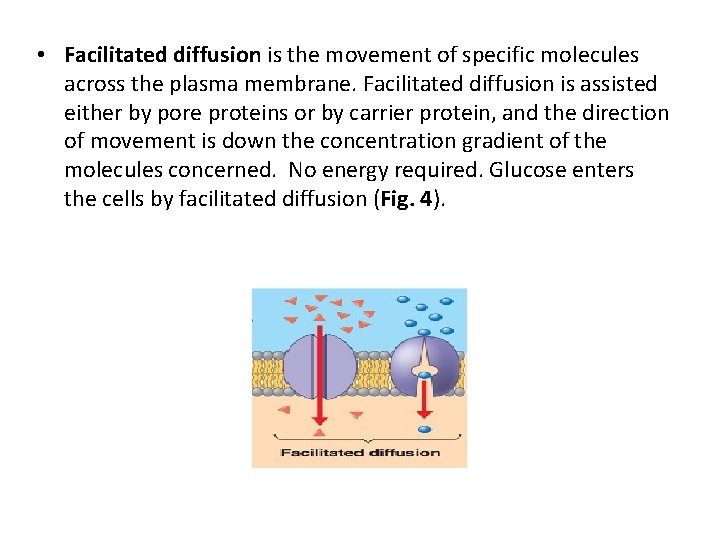
• Facilitated diffusion is the movement of specific molecules across the plasma membrane. Facilitated diffusion is assisted either by pore proteins or by carrier protein, and the direction of movement is down the concentration gradient of the molecules concerned. No energy required. Glucose enters the cells by facilitated diffusion (Fig. 4).
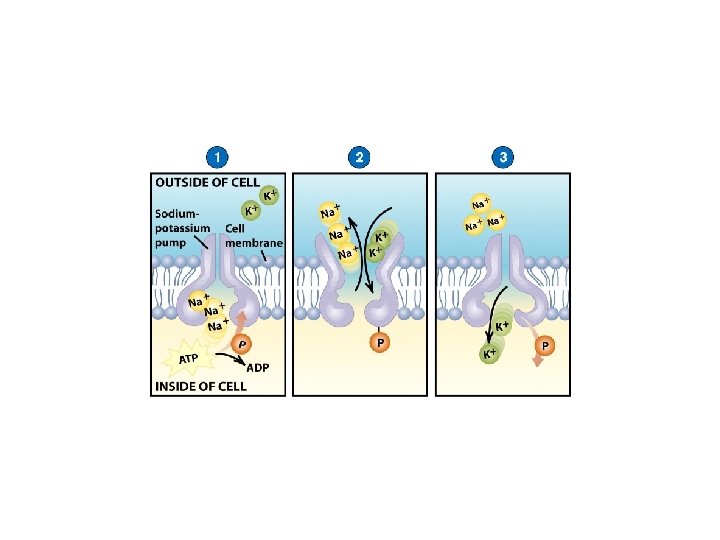
• Active transport is the movement of molecules from a region of lower concentration to a region of higher concentration. • Because this movement is happening against the concentration gradient, the cell must expend energy that is usually derived from a substance called adenosine triphosphate (ATP). • For example the sodium-potassium pumps in nerve cells. Na+ is maintained at low concentrations inside the cell and K+ is at higher concentrations (Fig. 5).
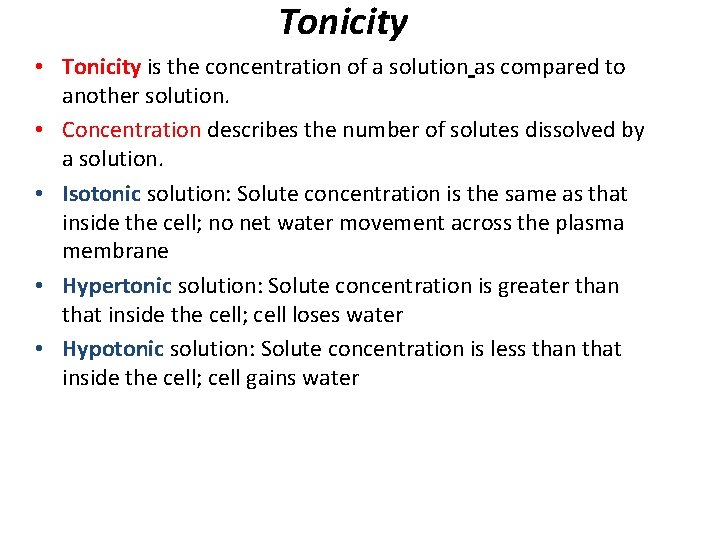
Tonicity • Tonicity is the concentration of a solution as compared to another solution. • Concentration describes the number of solutes dissolved by a solution. • Isotonic solution: Solute concentration is the same as that inside the cell; no net water movement across the plasma membrane • Hypertonic solution: Solute concentration is greater than that inside the cell; cell loses water • Hypotonic solution: Solute concentration is less than that inside the cell; cell gains water
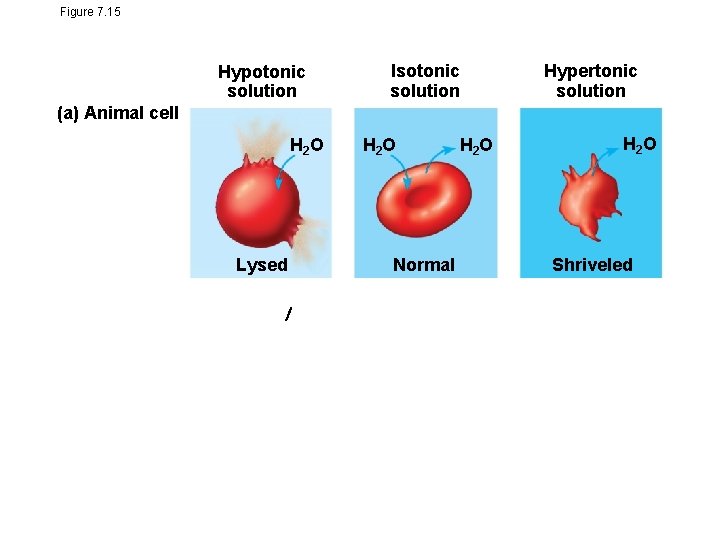
Figure 7. 15 Hypotonic solution Isotonic solution Hypertonic solution (a) Animal cell H 2 O Lysed H 2 O Normal Osmosis H 2 O Shriveled
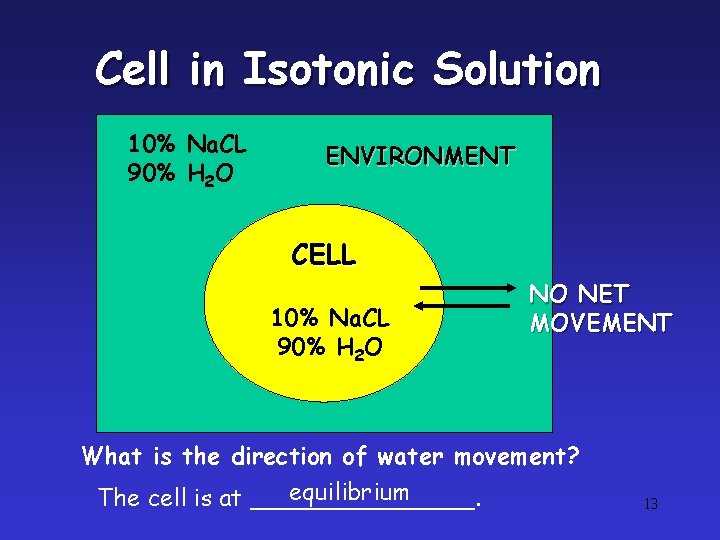
Cell in Isotonic Solution 10% Na. CL 90% H 2 O ENVIRONMENT CELL 10% Na. CL 90% H 2 O NO NET MOVEMENT What is the direction of water movement? equilibrium The cell is at ________. 13
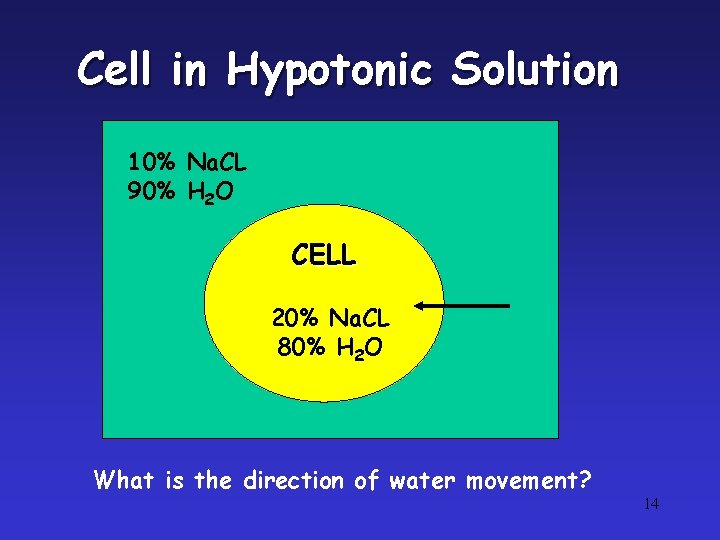
Cell in Hypotonic Solution 10% Na. CL 90% H 2 O CELL 20% Na. CL 80% H 2 O What is the direction of water movement? 14
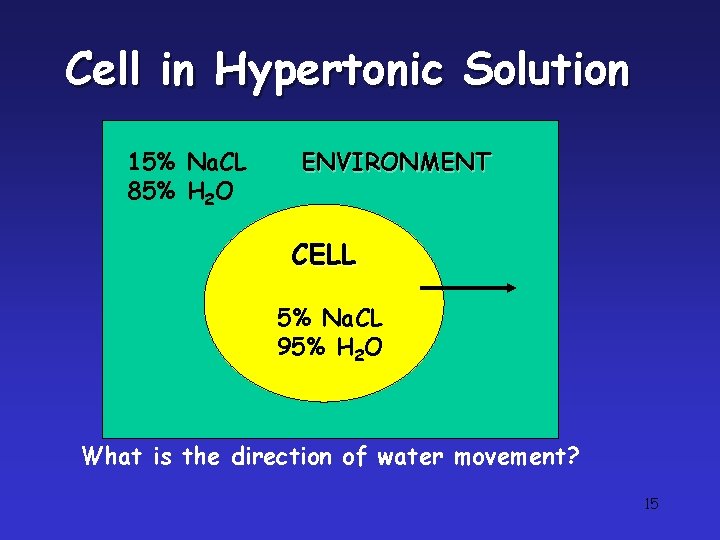
Cell in Hypertonic Solution 15% Na. CL 85% H 2 O ENVIRONMENT CELL 5% Na. CL 95% H 2 O What is the direction of water movement? 15
- Slides: 15