Transmutation Transmutation is the process of atoms of

- Slides: 15

Transmutation • Transmutation is the process of atoms of unstable nuclide A changing into atoms of nuclide B. – This can occur naturally (by radioactive decay) or. . . –. . . as a result of bombardment reactions • Example: Bombardment of nitrogen-14 with alpha particles. . . oxygen-17 was formed.

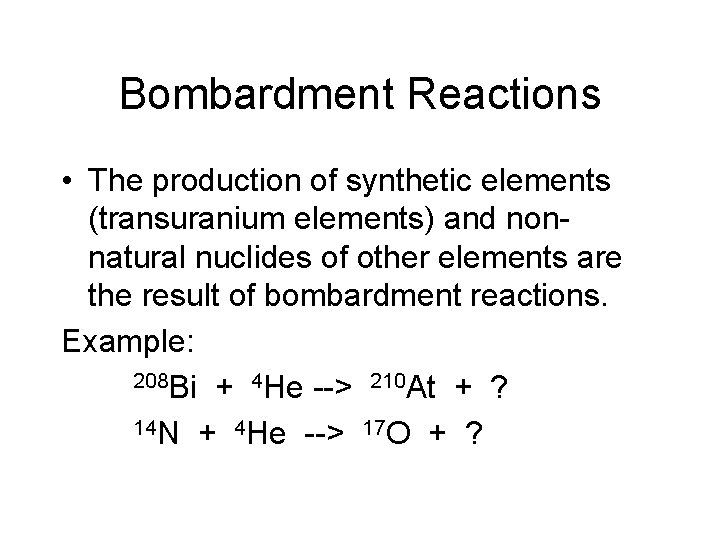
Bombardment Reactions • The production of synthetic elements (transuranium elements) and nonnatural nuclides of other elements are the result of bombardment reactions. Example: 208 Bi + 4 He --> 210 At + ? 14 N + 4 He --> 17 O + ?

Chemical Effects of Radiation • Radiations produced from radioactive decay can interact with atoms and molecules of surrounding material. – Electrons of these atoms/molecules are most directly affected by radiation. • Excitation • Ionization • Types of radiation: – Nonionizing - excitation (low energy) – Ionizing (high energy) - resulting in formation of ion pairs

Free Radical Formation – Free radical: atom, molecule or ion containing an unpaired electron – Free radicals rapidly react with nearby chemicals. • Stable molecule • Another free radical • Example: – H 2 O + radiation --> H 2 O+ + e– H 2 O+ + H 2 O --> H 3 O+ + OH (hydroxyl radical)

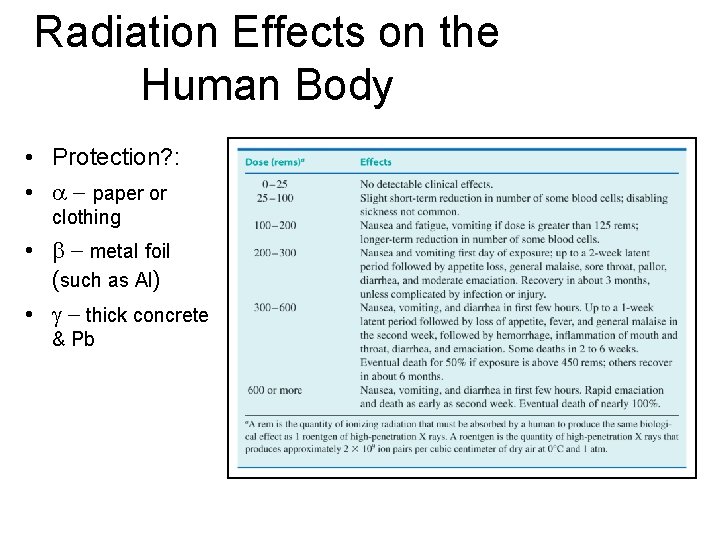
Radiation Effects on the Human Body • Protection? : • paper or clothing • metal foil (such as Al) • thick concrete & Pb

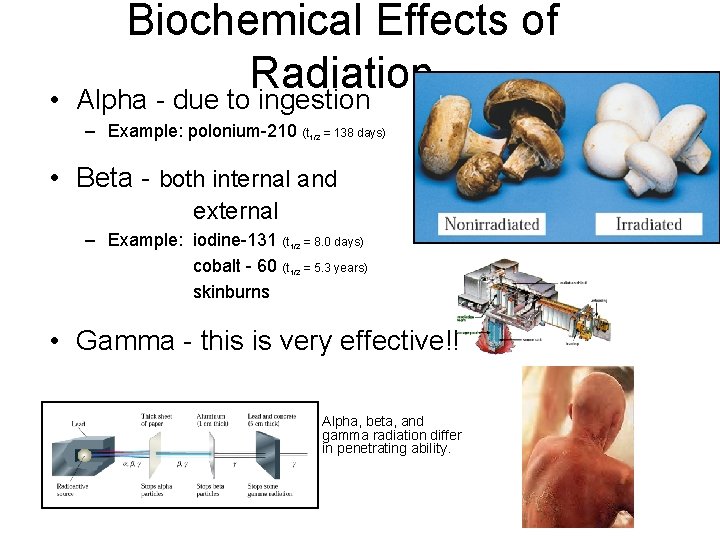
• Biochemical Effects of Radiation Alpha - due to ingestion – Example: polonium-210 (t 1/2 = 138 days) • Beta - both internal and external – Example: iodine-131 (t 1/2 = 8. 0 days) cobalt - 60 (t 1/2 = 5. 3 years) skinburns • Gamma - this is very effective!! Alpha, beta, and gamma radiation differ in penetrating ability.

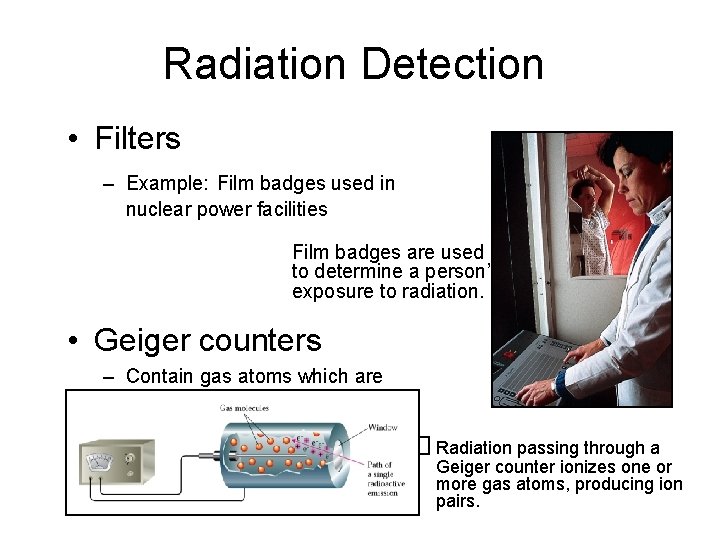
Radiation Detection • Filters – Example: Film badges used in nuclear power facilities Film badges are used to determine a person’s exposure to radiation. • Geiger counters – Contain gas atoms which are ionized. . . � Radiation passing through a Geiger counter ionizes one or more gas atoms, producing ion pairs.

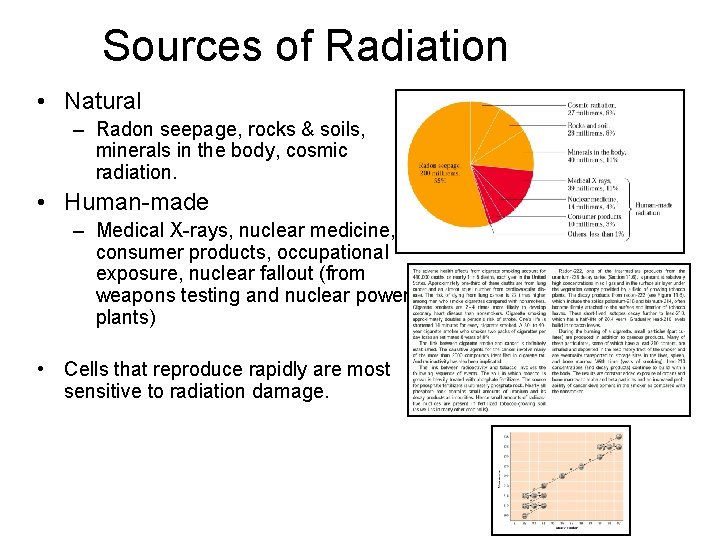
Sources of Radiation • Natural – Radon seepage, rocks & soils, minerals in the body, cosmic radiation. • Human-made – Medical X-rays, nuclear medicine, consumer products, occupational exposure, nuclear fallout (from weapons testing and nuclear power plants) • Cells that reproduce rapidly are most sensitive to radiation damage.

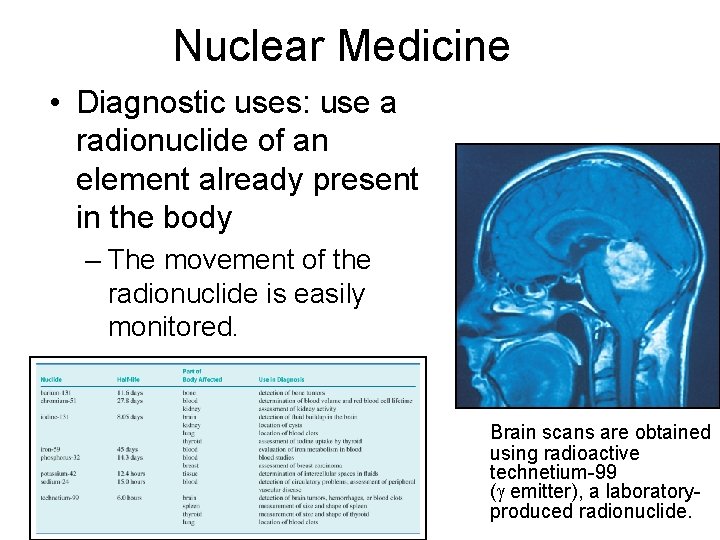
Nuclear Medicine • Diagnostic uses: use a radionuclide of an element already present in the body – The movement of the radionuclide is easily monitored. Brain scans are obtained using radioactive technetium-99 ( emitter), a laboratoryproduced radionuclide.

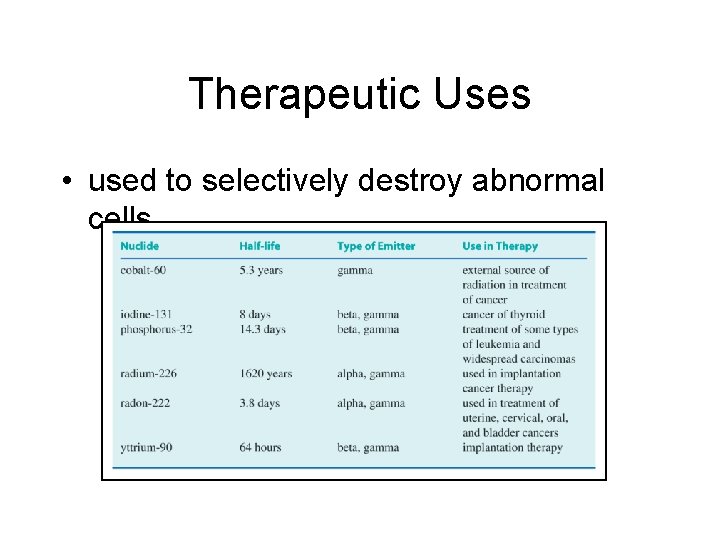
Therapeutic Uses • used to selectively destroy abnormal cells

Fission & Fusion reactions • Fission: a large nucleus splits into two mediumsized nuclei with the release of several free neutrons and LOTS of energy. • Fusion: two small nuclei are collided together to produce a larger nucleus and LOTS of energy.

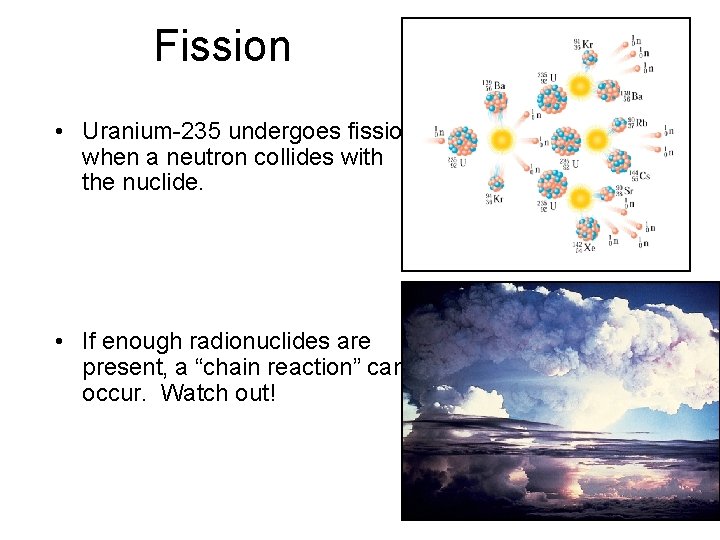
Fission • Uranium-235 undergoes fission when a neutron collides with the nuclide. • If enough radionuclides are present, a “chain reaction” can occur. Watch out!

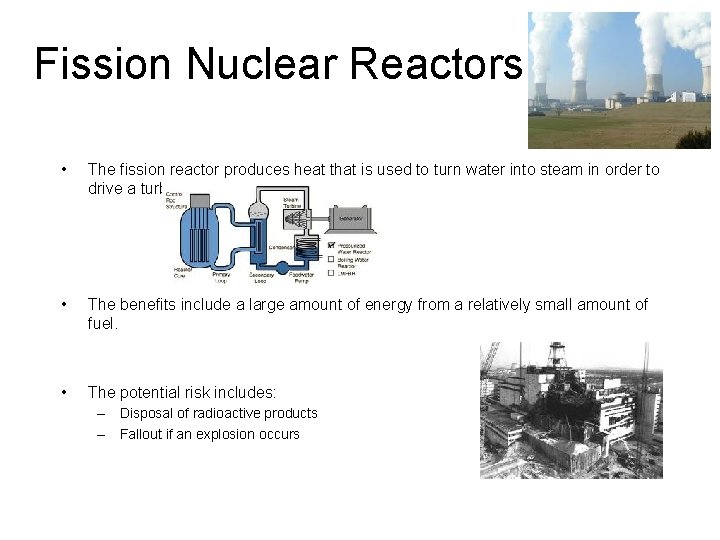
Fission Nuclear Reactors • The fission reactor produces heat that is used to turn water into steam in order to drive a turbine. • The benefits include a large amount of energy from a relatively small amount of fuel. • The potential risk includes: – Disposal of radioactive products – Fallout if an explosion occurs

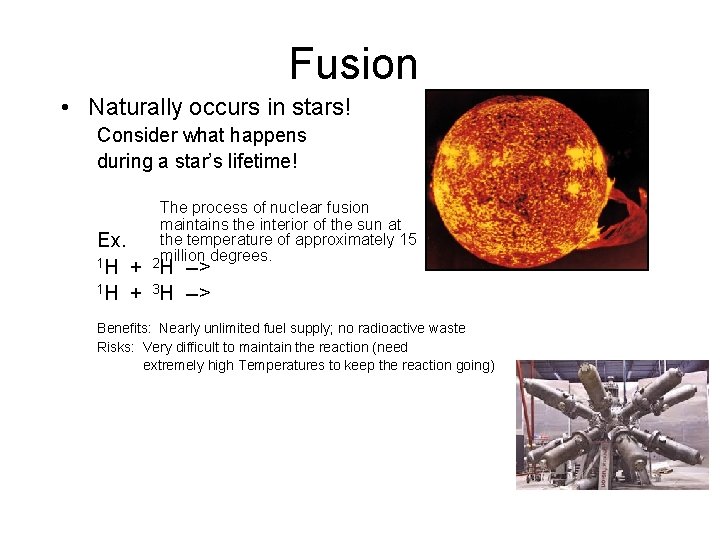
Fusion • Naturally occurs in stars! Consider what happens during a star’s lifetime! The process of nuclear fusion maintains the interior of the sun at the temperature of approximately 15 million degrees. 2 Ex. 1 H + H --> 1 H + 3 H --> Benefits: Nearly unlimited fuel supply; no radioactive waste Risks: Very difficult to maintain the reaction (need extremely high Temperatures to keep the reaction going)

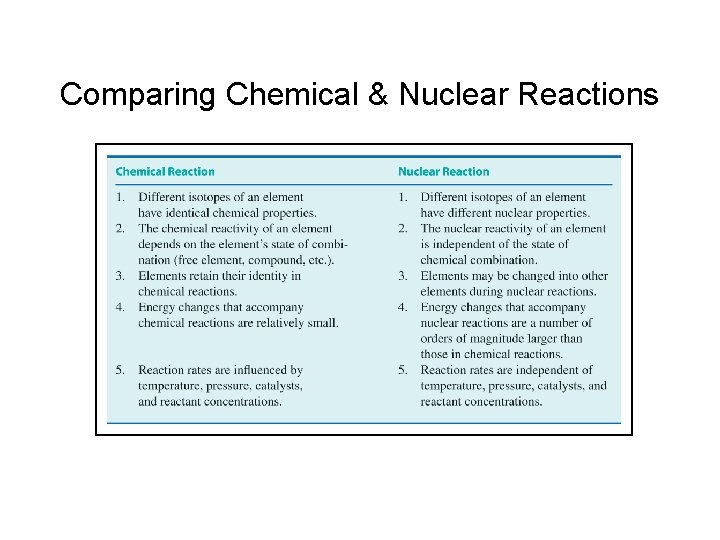
Comparing Chemical & Nuclear Reactions