Translation Step 2 of Protein Synthesis Molecular Components


- Slides: 14

Translation Step 2 of Protein Synthesis

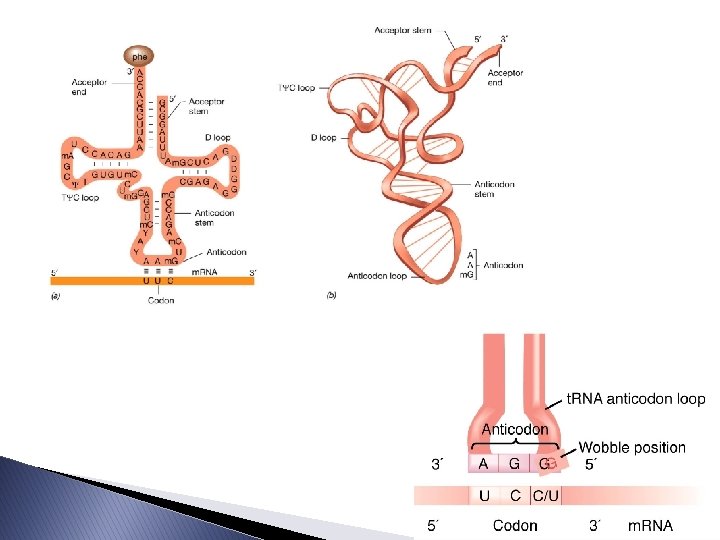
Molecular Components � Transfer RNA: takes amino acids from the cytoplasm to a ribosome � Each t. RNA carries a specific amino acid at one end and has an anticodon on the other end. � Anticodon: a nucleotide triplet which basepairs with a complementary codon on m. RNA. � t. RNA is a translator because it reads a nucleic acid word (m. RNA codon) and interprets it as a protein word (amino acid).

Molecular Components � The structure and function of t. RNA: ◦ Job – to take a specific amino acid to the ribosome, then go pick up another from the cytosol ◦ Looks like a cloverleaf if flattened into a 2 D structure due to the H-bonding between bases ◦ Its 3 D shape is roughly an “L. ” ◦ An enzyme called aminoacyl-t. RNA synthetase attaches a given amino acid to the appropriate t. RNA. There is a different synthetase for each amino acid. ◦ t. RNAs can sometimes match multiple codons because of the third base pair not needing to match exactly (“wobble”)


Molecular Components � Ribosomes ◦ Facilitate the coupling of t. RNA anticodons with m. RNA codons during protein synthesis ◦ 2 subunits: large and small ◦ Made of proteins and r. RNA (ribosomal RNA) ◦ Cells contain thousands of ribosomes, so r. RNA is the most abundant type of RNA. ◦ Since prokaryotic ribosomes are different from eukaryotic ribosomes, antibiotic drugs are able to inactivate them without inhibiting the ability of eukaryotic cells to make proteins.

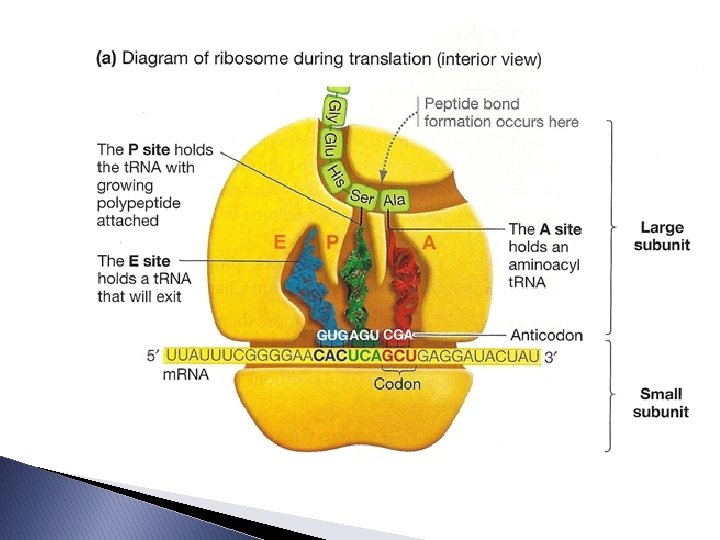
Molecular Components � Ribosomes ◦ Each has 3 binding sites: ◦ P site: (peptidyl-t. RNA site) holds the t. RNA carrying the growing polypeptide chain ◦ A site: (aminoacyl-t. RNA site) holds the t. RNA carrying the next amino acid to be added to the chain ◦ E site: (exit site) where discharged t. RNAs leave the ribosome ◦ The ribosome forms peptide bonds between the amino acids.


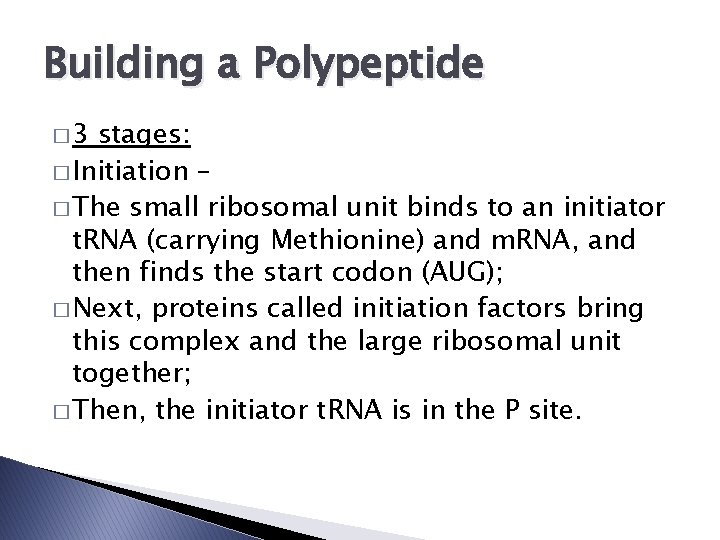
Building a Polypeptide � 3 stages: � Initiation – � The small ribosomal unit binds to an initiator t. RNA (carrying Methionine) and m. RNA, and then finds the start codon (AUG); � Next, proteins called initiation factors bring this complex and the large ribosomal unit together; � Then, the initiator t. RNA is in the P site.

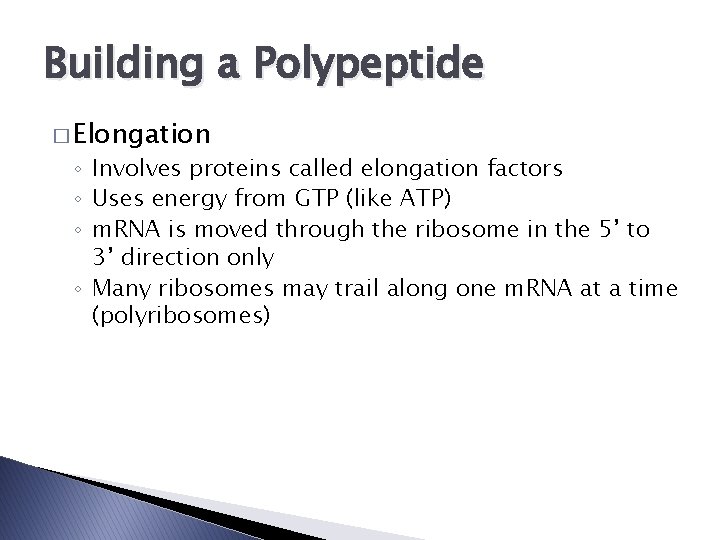
Building a Polypeptide � Elongation ◦ Involves proteins called elongation factors ◦ Uses energy from GTP (like ATP) ◦ m. RNA is moved through the ribosome in the 5’ to 3’ direction only ◦ Many ribosomes may trail along one m. RNA at a time (polyribosomes)

Building a Polypeptide � Termination ◦ Stop codon reaches the A site (triplets do not code for amino acids) ◦ A protein called a release factor binds to the stop codon and causes the addition of a water molecule instead of an amino acid ◦ The polypeptide is released ◦ Translation assembly comes apart

Completing and Targeting the Functional Protein � Protein Folding and Post-translational modifications: ◦ A protein may begin to coil and fold spontaneously. ◦ Chaperone proteins (“chaperonins”) usually help the polypeptide fold correctly. ◦ Examples of post-translational modifications before the protein can do its job… attachment of sugars, lipids, phosphate groups; removal of amino acids from the leading end; enzymatic cleaving into 2 or more pieces

Completing and Targeting the Functional Protein � Targeting Polypeptides to Specific Locations: ◦ 2 kinds of ribosomes – ◦ Free: suspended in cytosol and mostly synthesize proteins that dissolve in the cytosol and function there ◦ Bound: attached to the cytosolic side of the endoplasmic reticulum or nuclear envelope; make proteins of the endomembrane system as well as proteins secreted from the cell

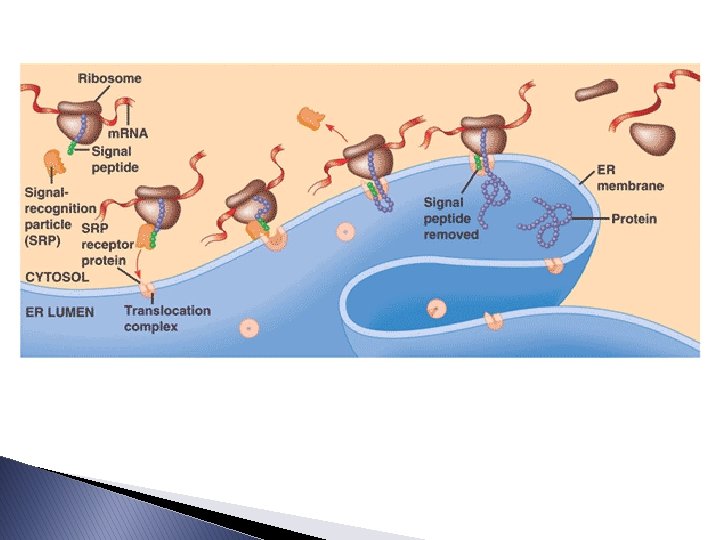
Completing and Targeting the Functional Protein � Targeting Polypeptides to Specific Locations: ◦ The above ribosomes are identical and can switch their status from free to bound. ◦ Polypeptide synthesis always starts in the cytosol with a free ribosome. ◦ If a protein that are destined for the endomembrane system are marked by a signal peptide. ◦ As it emerges from the ribosome, a signalrecognition particle (SRP) recognizes it and brings the ribosome to a receptor protein built into the ER.
