Transition Metals Definitions Complex A metal ion surrounded



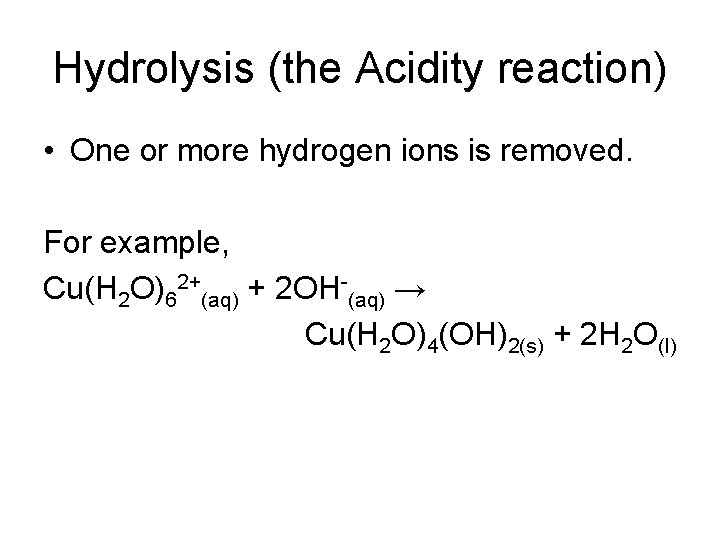

- Slides: 21

Transition Metals

Definitions • Complex: A metal ion surrounded by ligands. • Ligand: an electron pair donor i. e. a molecule or ion joined onto the metal ion by a dative covalent bond to the metal. • Coordination number: The number of atoms directly joined to a transition metal by a coordinate (dative covalent) bond.

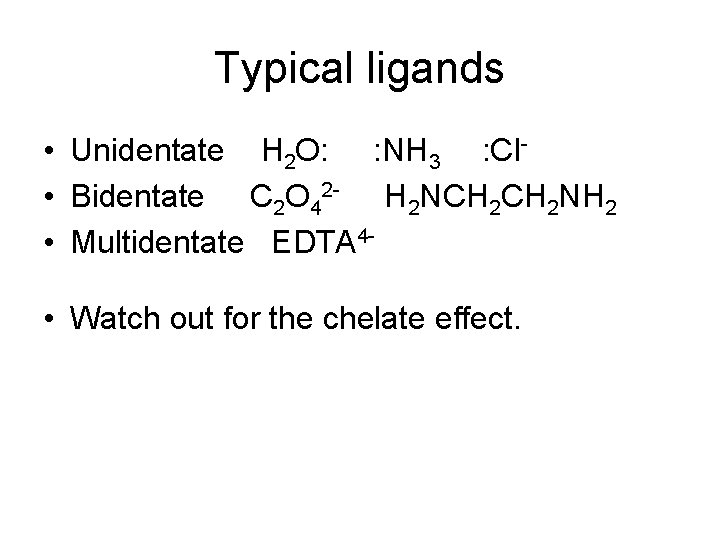
Typical ligands • Unidentate H 2 O: : NH 3 : Cl • Bidentate C 2 O 42 - H 2 NCH 2 NH 2 • Multidentate EDTA 4 • Watch out for the chelate effect.

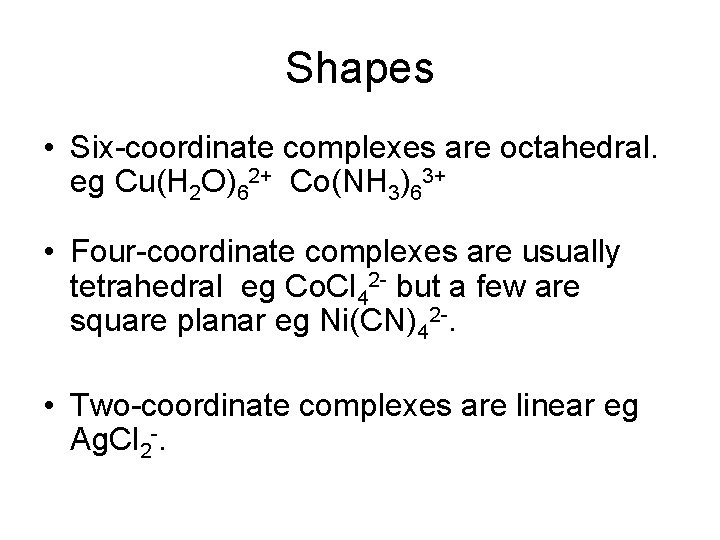
Shapes • Six-coordinate complexes are octahedral. eg Cu(H 2 O)62+ Co(NH 3)63+ • Four-coordinate complexes are usually tetrahedral eg Co. Cl 42 - but a few are square planar eg Ni(CN)42 -. • Two-coordinate complexes are linear eg Ag. Cl 2 -.

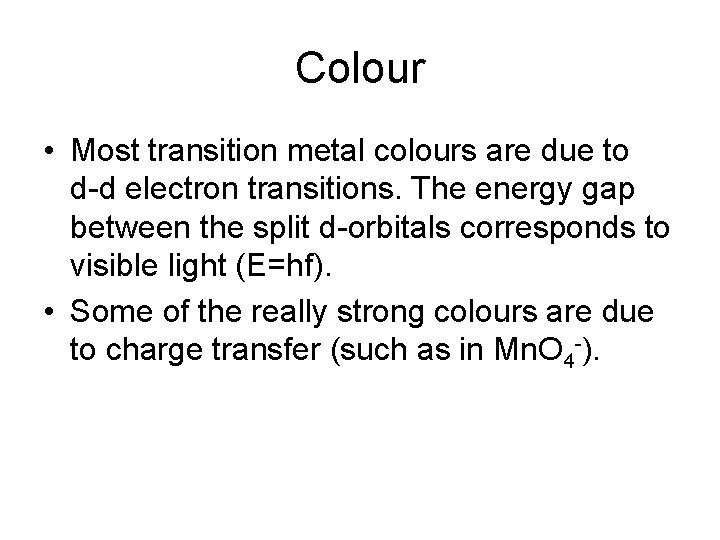
Colour • Most transition metal colours are due to d-d electron transitions. The energy gap between the split d-orbitals corresponds to visible light (E=hf). • Some of the really strong colours are due to charge transfer (such as in Mn. O 4 -).

Changing colour • Anything that changes the energy difference between the d-orbitals causes a change in colour: • Oxidation state • Ligand • Coordination number

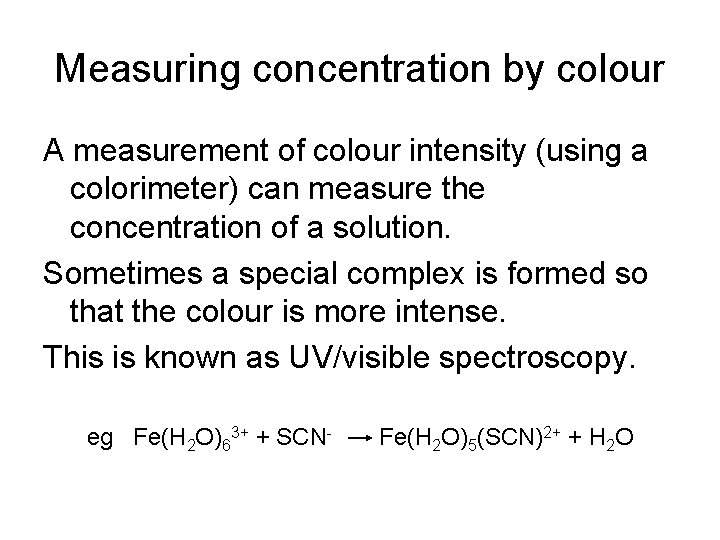
Measuring concentration by colour A measurement of colour intensity (using a colorimeter) can measure the concentration of a solution. Sometimes a special complex is formed so that the colour is more intense. This is known as UV/visible spectroscopy. eg Fe(H 2 O)63+ + SCN- Fe(H 2 O)5(SCN)2+ + H 2 O

Reaction types • The reactions of the transition metals come under three headings (but sometimes more than one occurs at the same time!). • Ligand substitution • Hydrolysis (the acidity reaction) • Redox

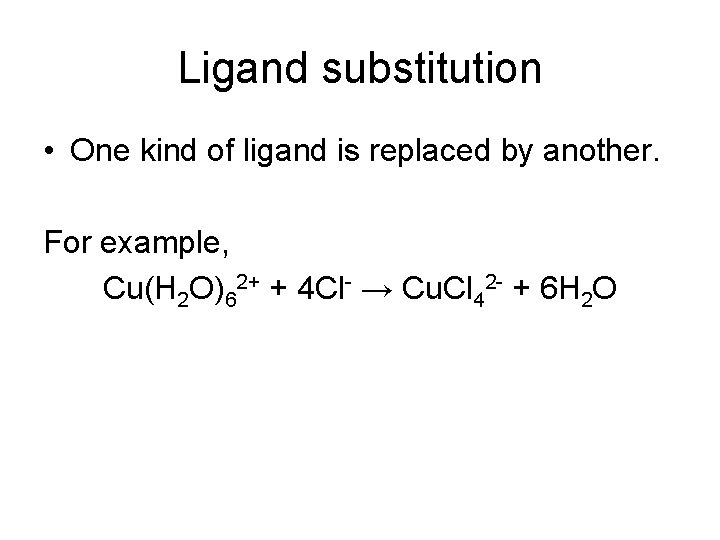
Ligand substitution • One kind of ligand is replaced by another. For example, Cu(H 2 O)62+ + 4 Cl- → Cu. Cl 42 - + 6 H 2 O
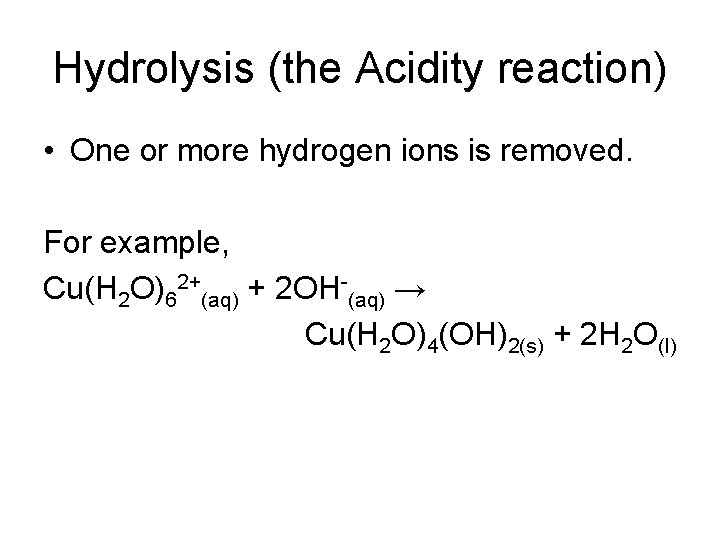
Hydrolysis (the Acidity reaction) • One or more hydrogen ions is removed. For example, Cu(H 2 O)62+(aq) + 2 OH-(aq) → Cu(H 2 O)4(OH)2(s) + 2 H 2 O(l)

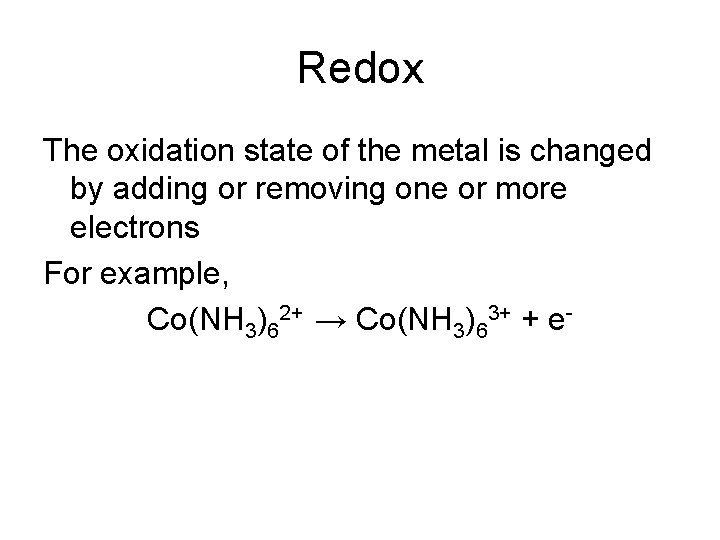
Redox The oxidation state of the metal is changed by adding or removing one or more electrons For example, Co(NH 3)62+ → Co(NH 3)63+ + e-

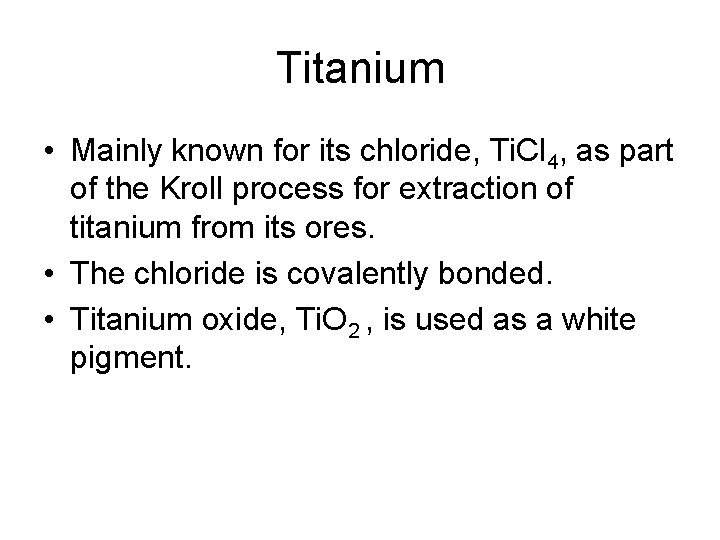
Titanium • Mainly known for its chloride, Ti. Cl 4, as part of the Kroll process for extraction of titanium from its ores. • The chloride is covalently bonded. • Titanium oxide, Ti. O 2 , is used as a white pigment.

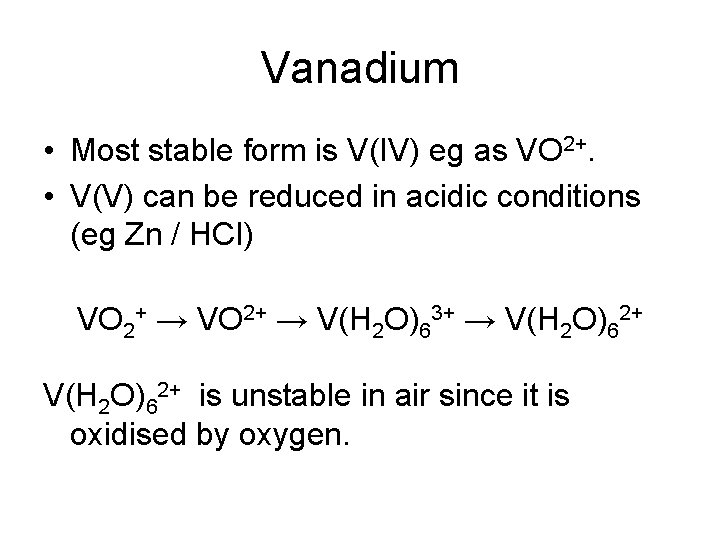
Vanadium • Most stable form is V(IV) eg as VO 2+. • V(V) can be reduced in acidic conditions (eg Zn / HCl) VO 2+ → V(H 2 O)63+ → V(H 2 O)62+ is unstable in air since it is oxidised by oxygen.

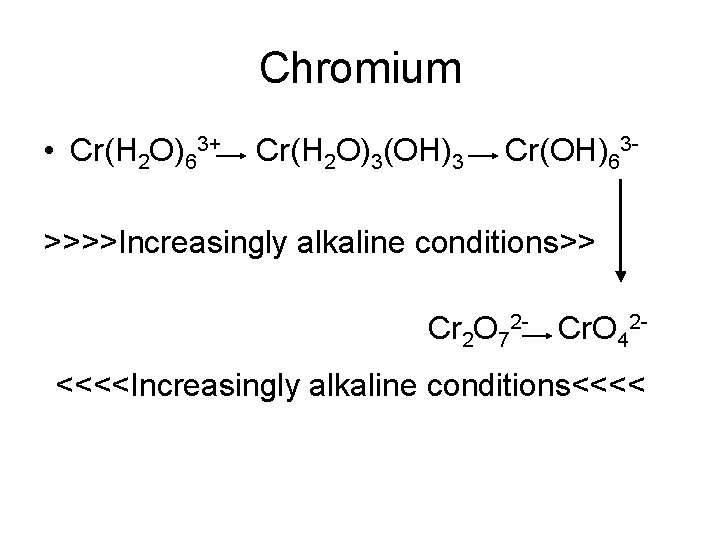
Chromium • Cr(H 2 O)63+ Cr(H 2 O)3(OH)3 Cr(OH)63 - >>>>Increasingly alkaline conditions>> Cr 2 O 72 - Cr. O 42<<<<Increasingly alkaline conditions<<<<

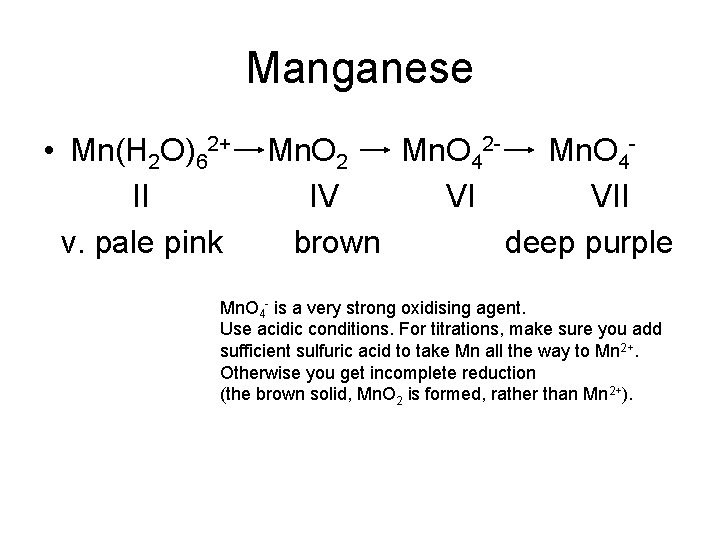
Manganese • Mn(H 2 O)62+ II v. pale pink Mn. O 2 Mn. O 42 - Mn. O 4 IV VI VII brown deep purple Mn. O 4 - is a very strong oxidising agent. Use acidic conditions. For titrations, make sure you add sufficient sulfuric acid to take Mn all the way to Mn 2+. Otherwise you get incomplete reduction (the brown solid, Mn. O 2 is formed, rather than Mn 2+).

Iron • Fe(H 2 O)62+ is non-acidic in water (green). • Pure Fe(H 2 O)63+ is a lilac colour but on contact with water goes rusty brown. • Fe(H 2 O)62+ forms Fe(OH)2 (a green solid) with Na. OH but it goes brown (forming Fe(OH)3 on standing in air.

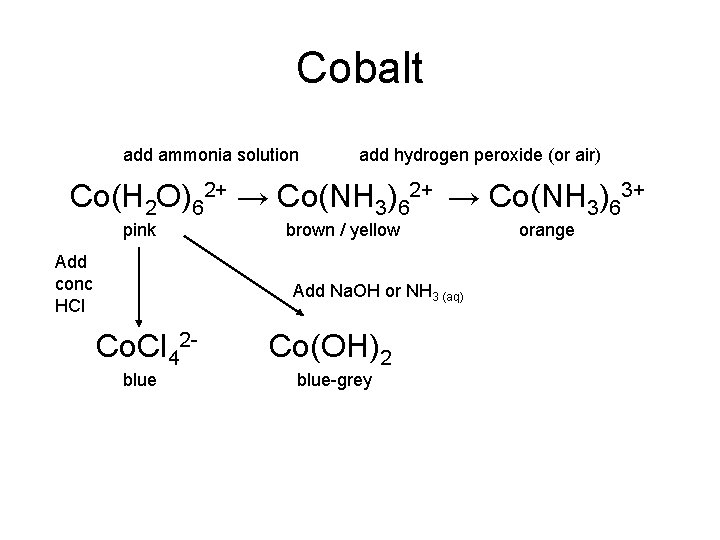
Cobalt add ammonia solution add hydrogen peroxide (or air) Co(H 2 O)62+ → Co(NH 3)63+ pink Add conc HCl brown / yellow Add Na. OH or NH 3 (aq) Co. Cl 42 blue Co(OH)2 blue-grey orange

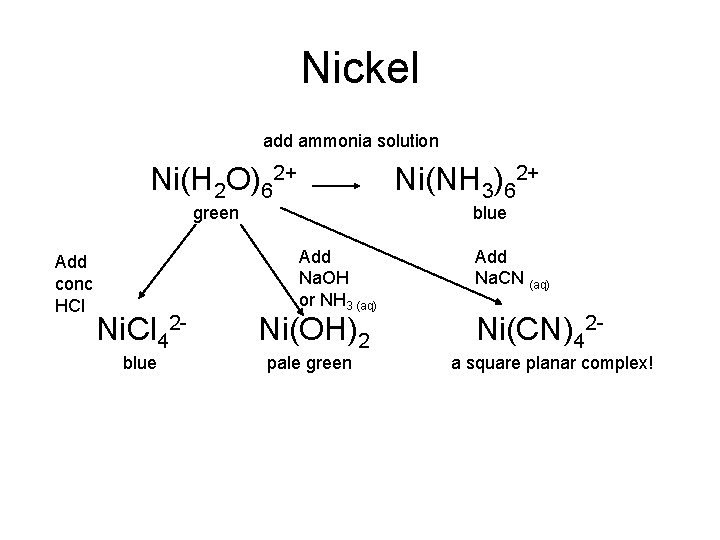
Nickel add ammonia solution Ni(H 2 O)62+ Ni(NH 3)62+ green Add conc HCl blue Add Na. OH or NH 3 (aq) Ni. Cl 42 - Ni(OH)2 blue pale green Add Na. CN (aq) Ni(CN)42 a square planar complex!

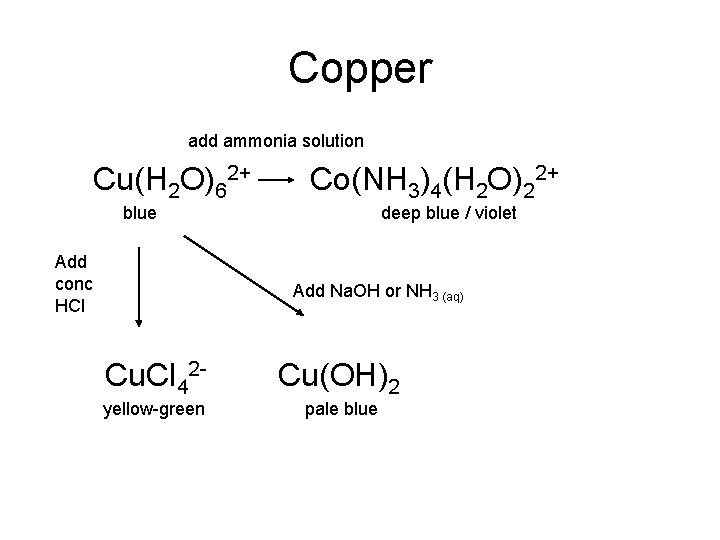
Copper add ammonia solution Cu(H 2 O)62+ Co(NH 3)4(H 2 O)22+ blue Add conc HCl deep blue / violet Add Na. OH or NH 3 (aq) Cu. Cl 42 - Cu(OH)2 yellow-green pale blue

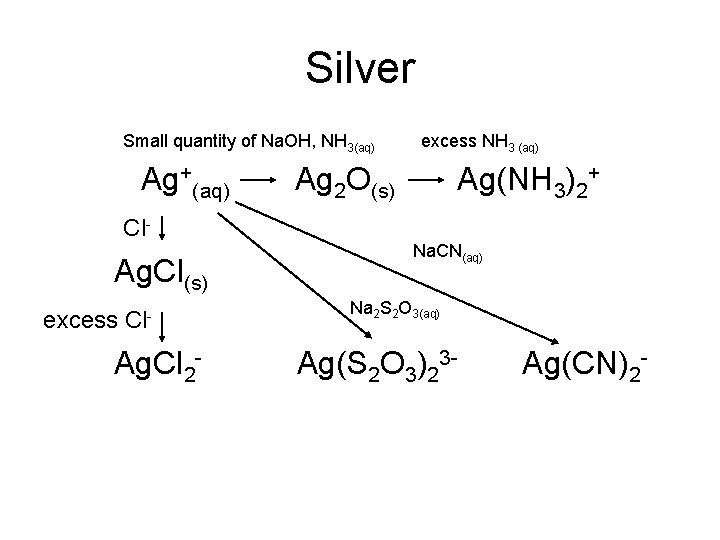
Silver Small quantity of Na. OH, NH 3(aq) Ag+(aq) Cl- Ag. Cl(s) excess Cl- Ag. Cl 2 - excess NH 3 (aq) Ag 2 O(s) Ag(NH 3)2+ Na. CN(aq) Na 2 S 2 O 3(aq) Ag(S 2 O 3)23 - Ag(CN)2 -

Uses of transition metals and their complexes • V 2 O 5 is used as a catalyst in the Contact Process (manufacture of sulfuric acid). • Cis-platin (a platinum complex) is a very effective anti-cancer drug. • Fe 2+ is an important part of haemoglobin. • Ag(NH 3)2+ is Tollen’s reagent (test for aldehydes). • Ag(S 2 O 3)23 - is formed during photographic processing. • Ag(CN)2 - is used in electroplating.