Transition metals are unusual because many of them


*Transition metals are unusual because many of them are able to form several different ions. *Iron has two different charge options, it can form Fe+2 or Fe+3 ions. *In order to know the charge of the metal, it has to be given in the name.
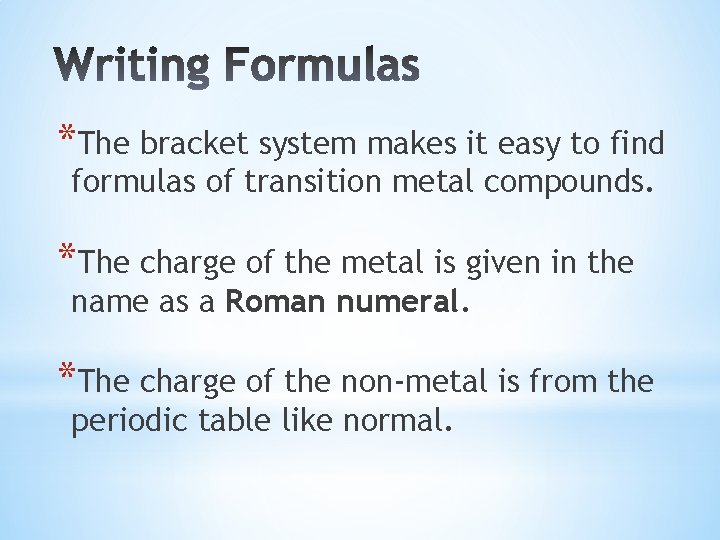
*The bracket system makes it easy to find formulas of transition metal compounds. *The charge of the metal is given in the name as a Roman numeral. *The charge of the non-metal is from the periodic table like normal.

*Example: Copper (II) chloride *The charge of the copper ion is +2 from the Roman number Cu+2 Cl-1 Cu. Cl 2 Crossover the numbers to get the formula

1. Nickel (III) sulfide Ni 2 S 3 2. Chromium (II) bromide Cr. Br 2 3. Manganese (IV) fluoride Mn. F 4 4. Copper (II) oxide Cu 2 O 2 reduces to Cu. O

*When we name a transition metal ionic compound, we MUST include the charge of the metal ion in brackets. *The charge is given as a Roman number.

Co 2 O 3 these two elements are cobalt and oxygen, so the name will be cobalt ( ) oxide Co 2 O 3 Reverse crossover the charges UP to see what the charge will be for each element Co+3 O-2 *the cobalt has a +3 charge *We need to check that the charge on the non-metal is correct. Oxygen does have a -2 charge. The name will be: Cobalt (III) oxide

1. Fe. O *Iron (II) oxide 2. Ni 2 O 3 *Nickel (III) oxide 3. Mn. Br 4 *Manganese (IV) bromide 4. Ni. O *Nickel (II) oxide 5. Au. F *Gold (I) fluoride
- Slides: 8