Transient Absorption Courtesy of Kenneth Hanson Florida University
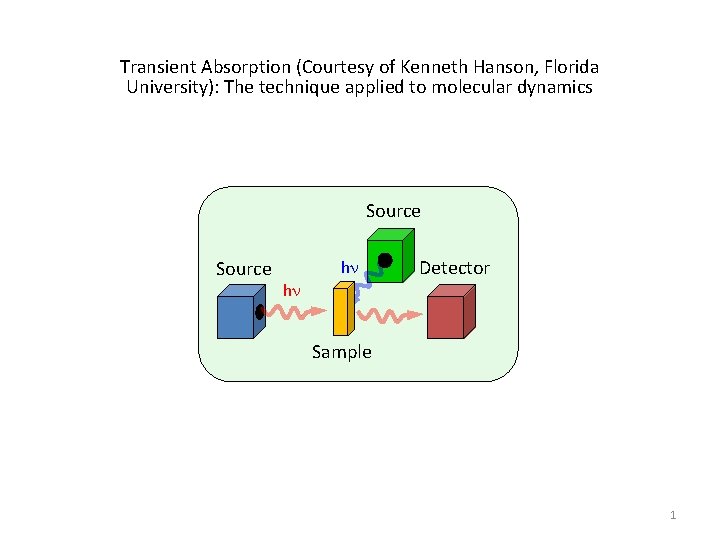
Transient Absorption (Courtesy of Kenneth Hanson, Florida University): The technique applied to molecular dynamics Source hn hn Detector Sample 1
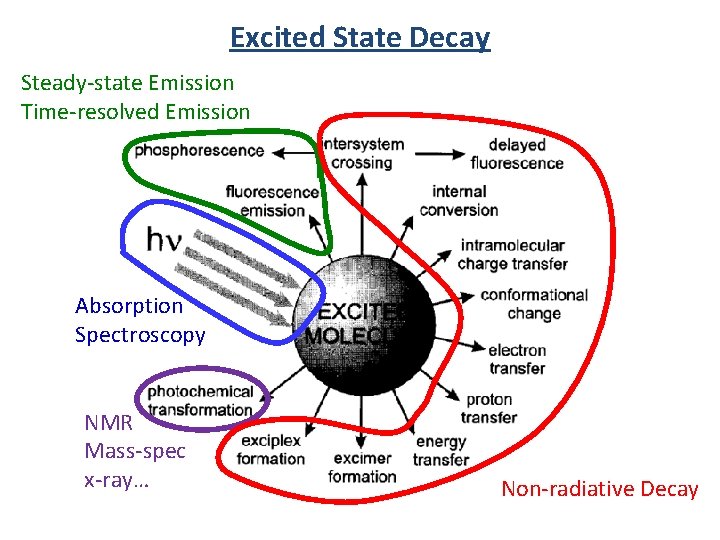
Excited State Decay Steady-state Emission Time-resolved Emission Absorption Spectroscopy NMR Mass-spec x-ray… Non-radiative Decay
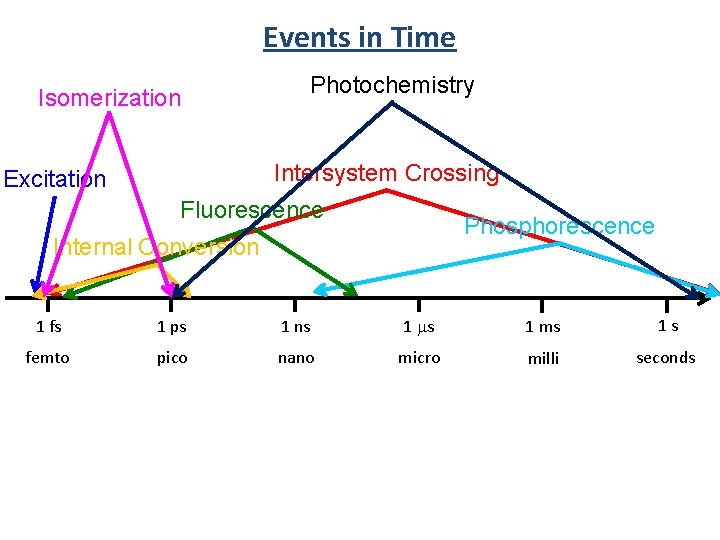
Events in Time Isomerization Photochemistry Intersystem Crossing Excitation Fluorescence Phosphorescence Internal Conversion 1 fs 1 ps 1 ns 1 ms 1 s femto pico nano micro milli seconds
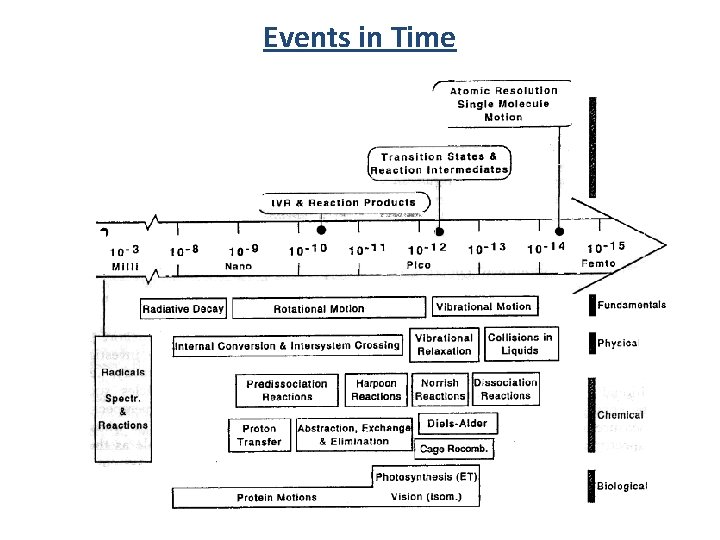
Events in Time
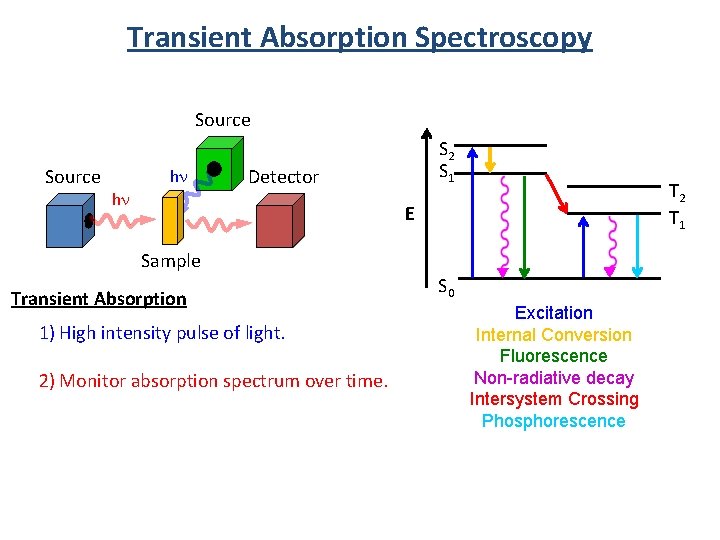
Transient Absorption Spectroscopy Source hn hn S 2 S 1 Detector T 2 T 1 E Sample Transient Absorption 1) High intensity pulse of light. 2) Monitor absorption spectrum over time. S 0 Excitation Internal Conversion Fluorescence Non-radiative decay Intersystem Crossing Phosphorescence
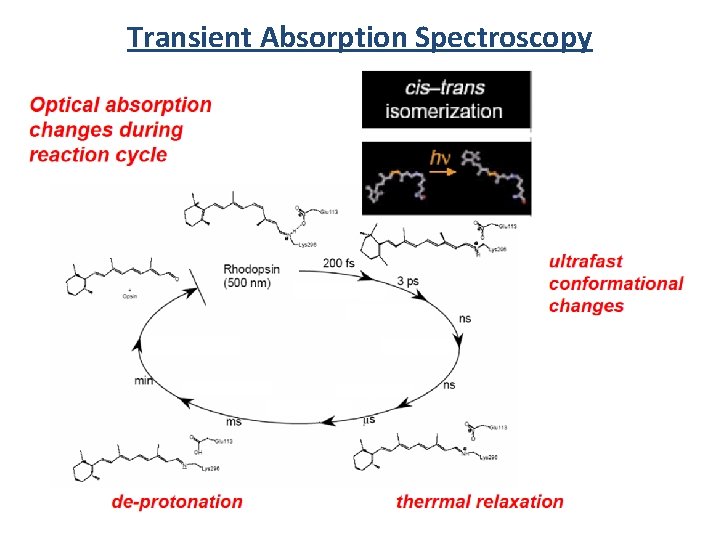
Transient Absorption Spectroscopy
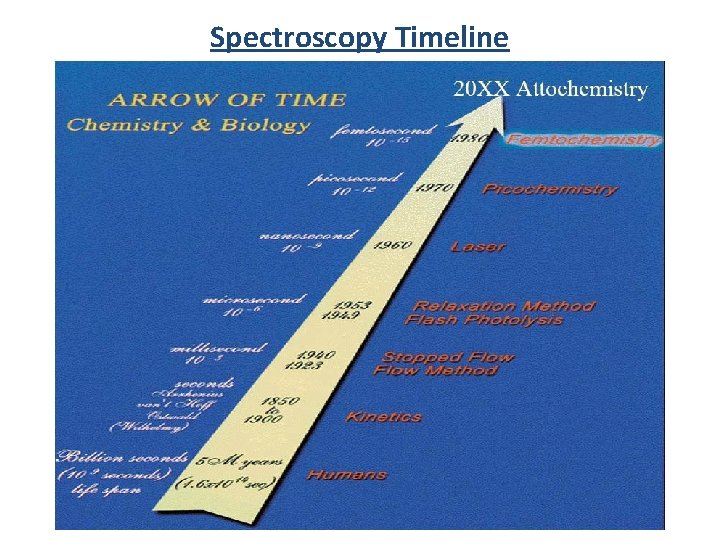
Spectroscopy Timeline
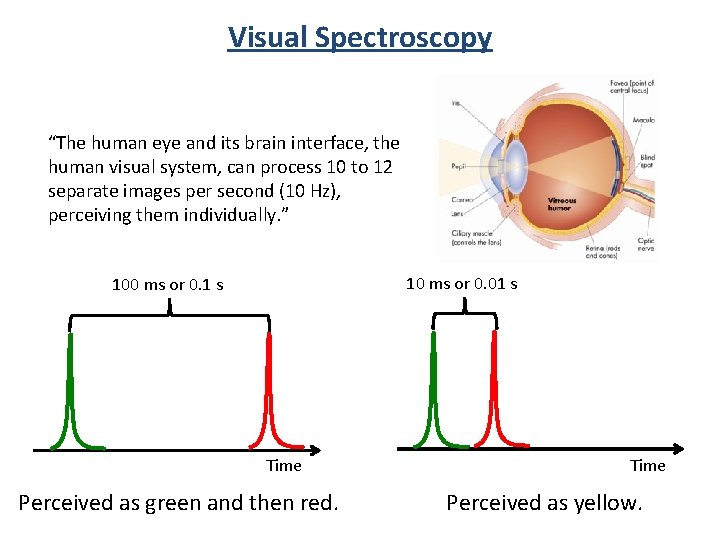
Visual Spectroscopy “The human eye and its brain interface, the human visual system, can process 10 to 12 separate images per second (10 Hz), perceiving them individually. ” 10 ms or 0. 01 s 100 ms or 0. 1 s Time Perceived as green and then red. Time Perceived as yellow.

We are missing out! 70 Hz 14 ms per cycle
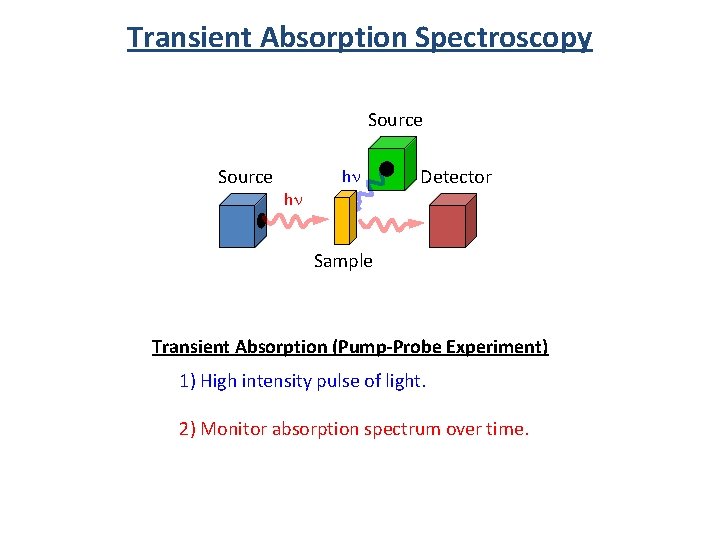
Transient Absorption Spectroscopy Source hn hn Detector Sample Transient Absorption (Pump-Probe Experiment) 1) High intensity pulse of light. 2) Monitor absorption spectrum over time.
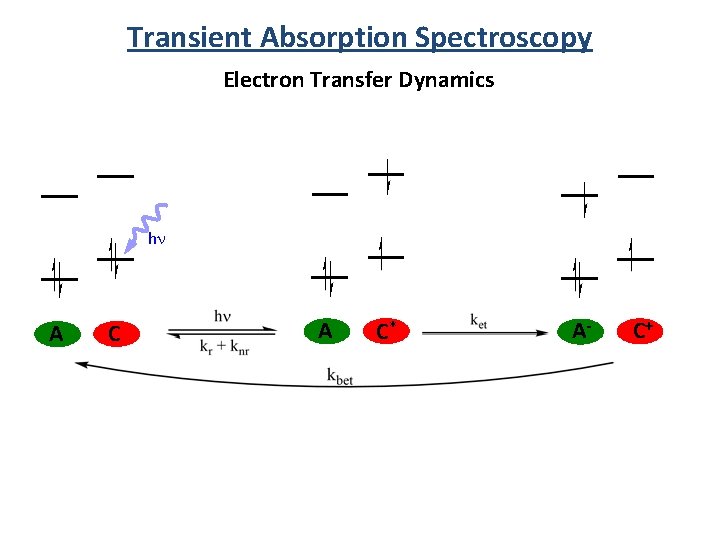
Transient Absorption Spectroscopy Electron Transfer Dynamics hn A C* A- C+
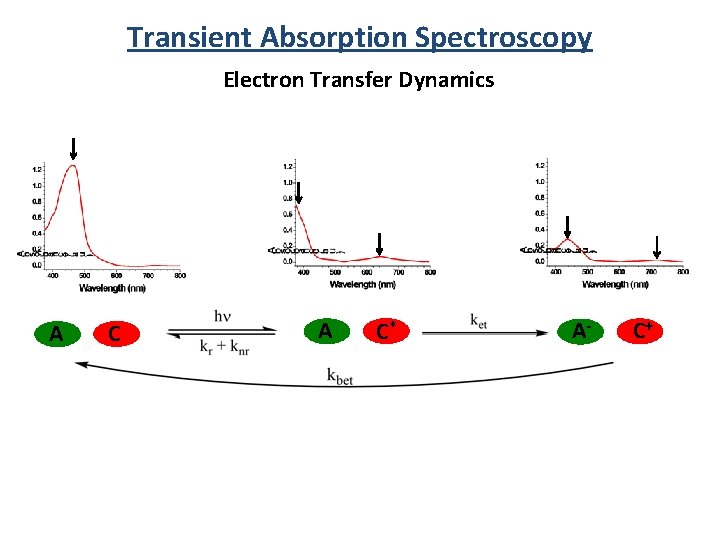
Transient Absorption Spectroscopy Electron Transfer Dynamics A C* A- C+
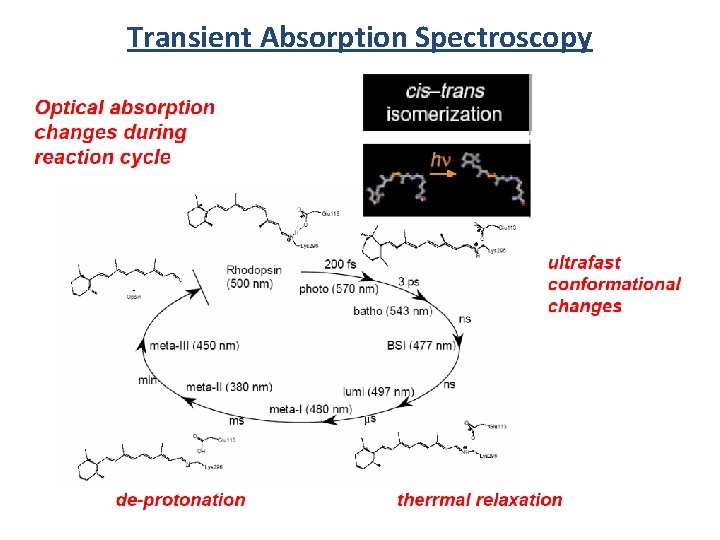
Transient Absorption Spectroscopy
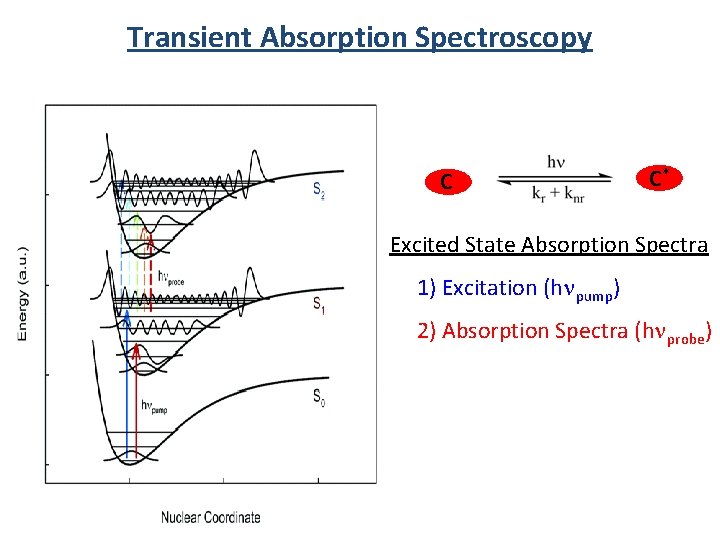
Transient Absorption Spectroscopy C C* Excited State Absorption Spectra 1) Excitation (hnpump) 2) Absorption Spectra (hnprobe)
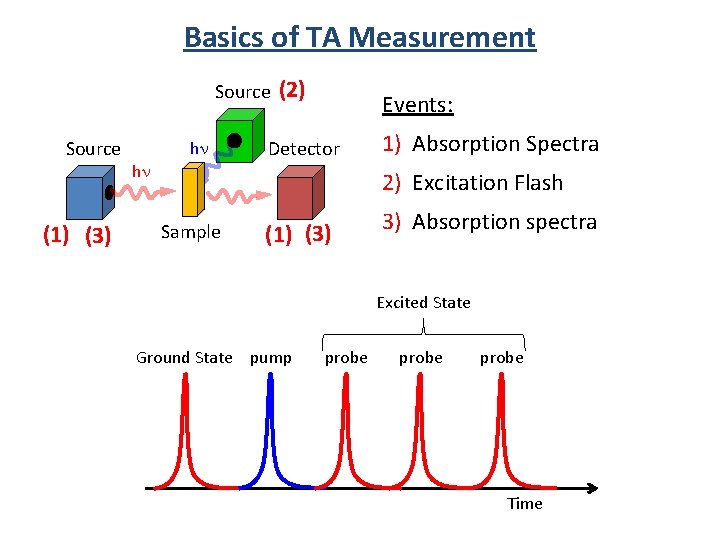
Basics of TA Measurement Source (1) (3) hn hn (2) Events: Detector 1) Absorption Spectra 2) Excitation Flash Sample (1) (3) 3) Absorption spectra Excited State Ground State pump probe Time
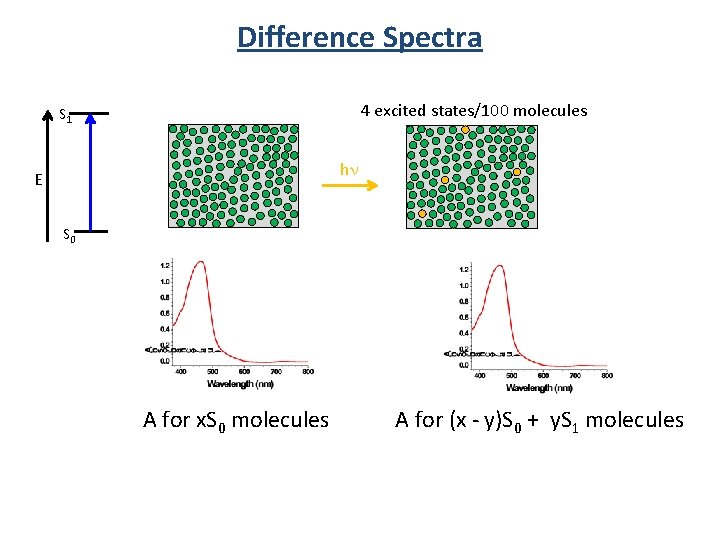
Difference Spectra 4 excited states/100 molecules S 1 hn E S 0 A for x. S 0 molecules A for (x - y)S 0 + y. S 1 molecules
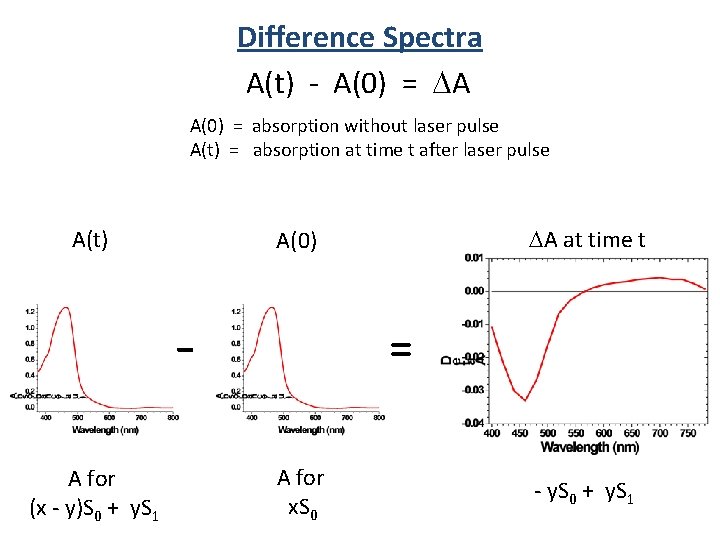
Difference Spectra A(t) - A(0) = A A(0) = absorption without laser pulse A(t) = absorption at time t after laser pulse A(t) A for (x - y)S 0 + y. S 1 A at time t A(0) = A for x. S 0 - y. S 0 + y. S 1
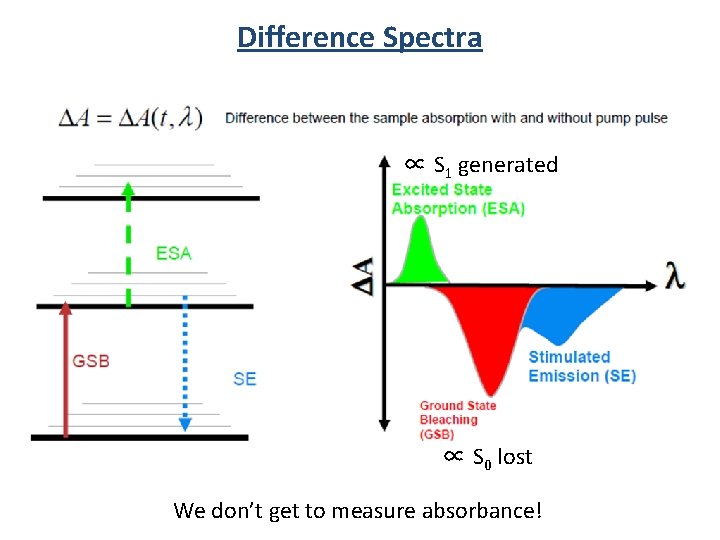
Difference Spectra ∝ S 1 generated ∝ S 0 lost We don’t get to measure absorbance!
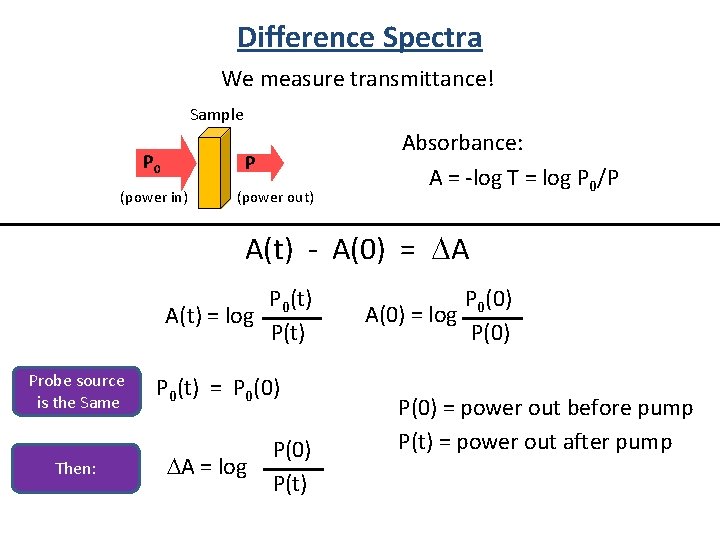
Difference Spectra We measure transmittance! Sample P 0 P (power in) (power out) Absorbance: A = -log T = log P 0/P A(t) - A(0) = A P 0(t) A(t) = log P(t) Probe source is the Same Then: P 0(t) = P 0(0) A = log P(0) P(t) P 0(0) A(0) = log P(0) = power out before pump P(t) = power out after pump
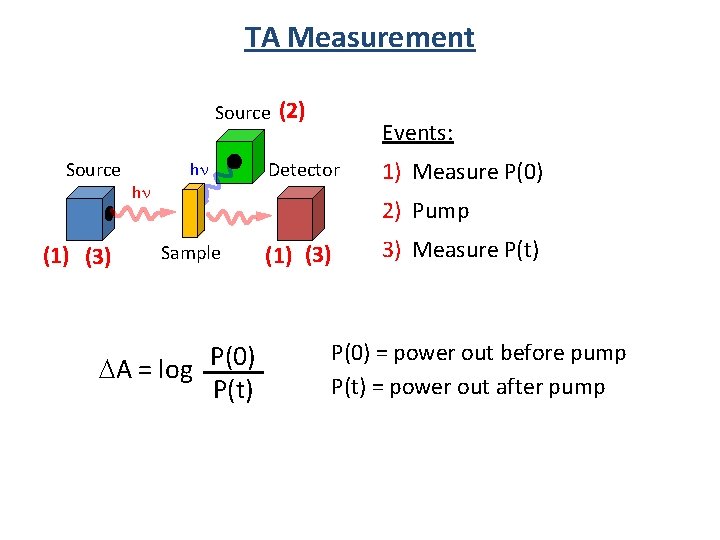
TA Measurement Source (1) (3) hn hn (2) Events: Detector 1) Measure P(0) 2) Pump Sample A = log P(0) P(t) (1) (3) 3) Measure P(t) P(0) = power out before pump P(t) = power out after pump
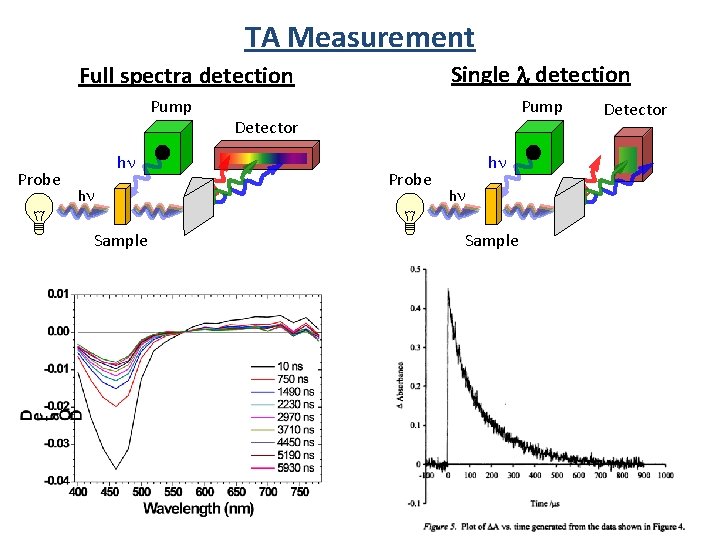
TA Measurement Single l detection Full spectra detection Pump Probe hn hn Sample Pump Detector Probe hn hn Sample Detector
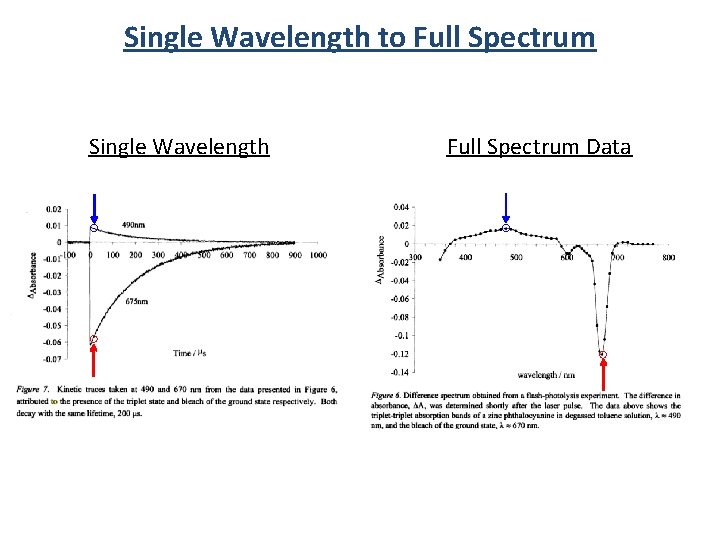
Single Wavelength to Full Spectrum Single Wavelength Full Spectrum Data
Femtosecond TA (10 -15 s) First developed in the 1980 s (A. H. Zewail) 1999 Nobel Prize in Chemistry “for his studies of the transition states of chemical reactions using femtosecond spectroscopy"
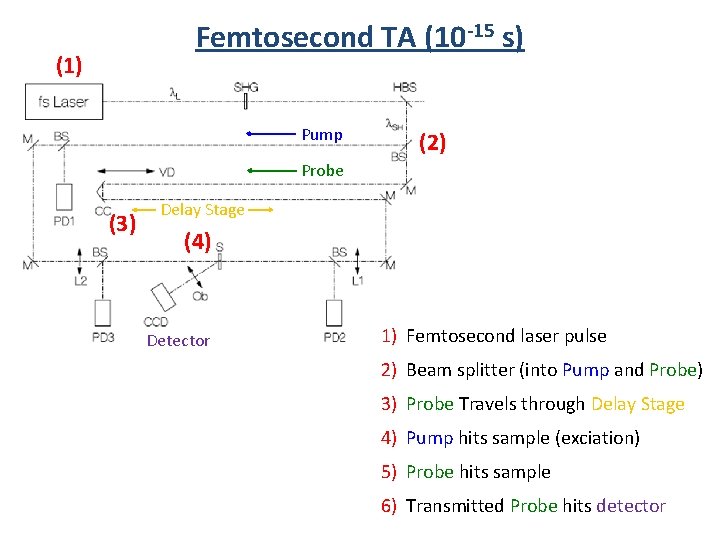
Femtosecond TA (10 -15 s) (1) Pump (2) Probe (3) Delay Stage (4) Detector 1) Femtosecond laser pulse 2) Beam splitter (into Pump and Probe) 3) Probe Travels through Delay Stage 4) Pump hits sample (exciation) 5) Probe hits sample 6) Transmitted Probe hits detector
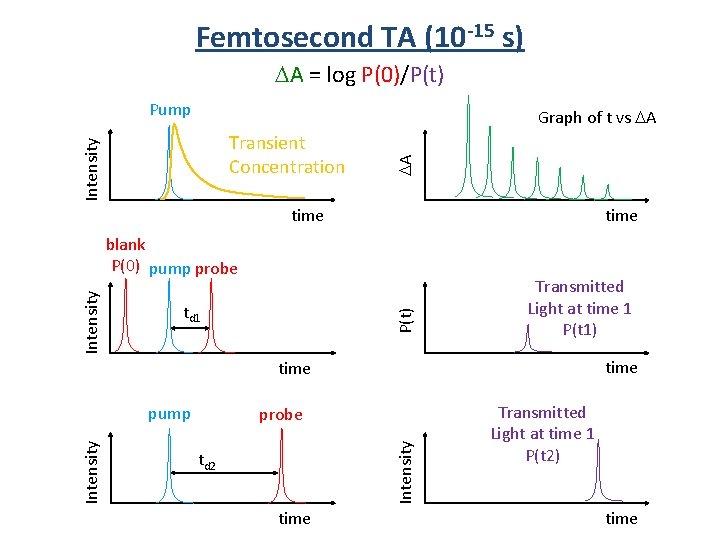
Femtosecond TA (10 -15 s) A = log P(0)/P(t) Pump Intensity Transient Concentration A Graph of t vs A time td 1 P(t) Intensity blank P(0) pump probe Transmitted Light at time 1 P(t 1) time probe Intensity pump td 2 time Transmitted Light at time 1 P(t 2) time
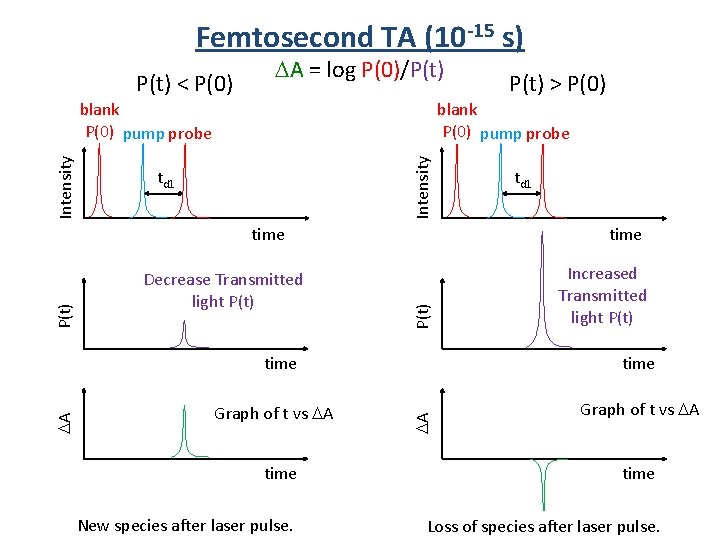
Femtosecond TA (10 -15 s) P(t) < P(0) A = log P(0)/P(t) blank P(0) pump probe Intensity blank P(0) pump probe td 1 Decrease Transmitted light P(t) time Increased Transmitted light P(t) time A Graph of t vs A td 1 time P(t) time A P(t) > P(0) Graph of t vs A time New species after laser pulse. Loss of species after laser pulse.
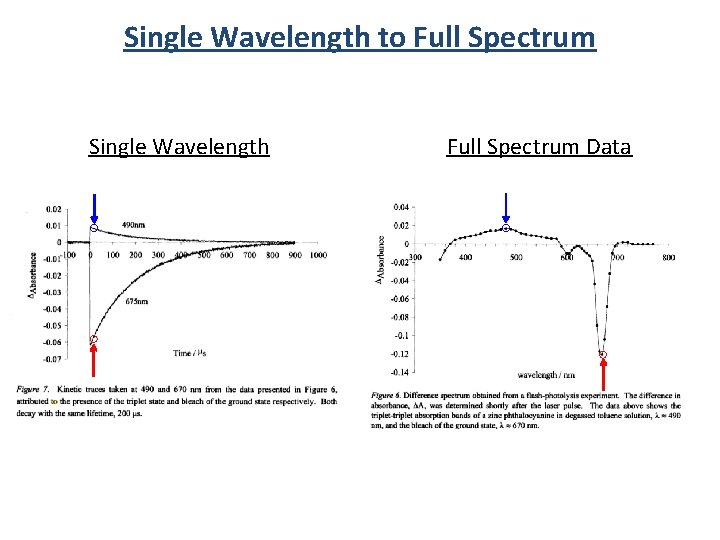
Single Wavelength to Full Spectrum Single Wavelength Full Spectrum Data
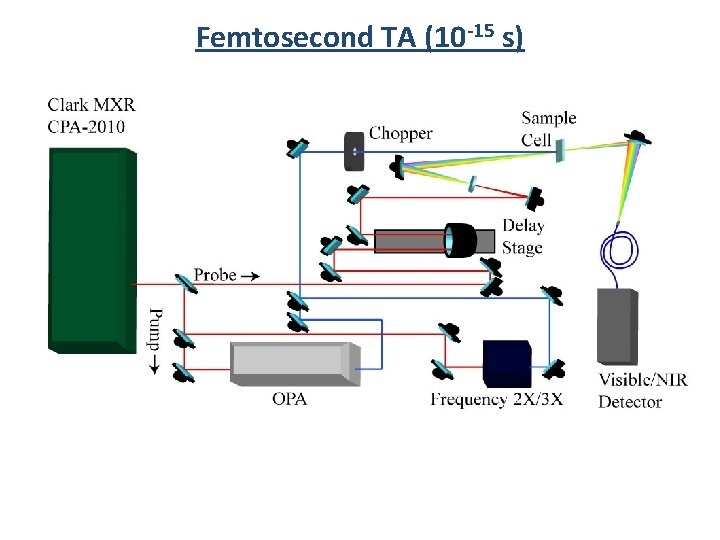
Femtosecond TA (10 -15 s)
A striking example
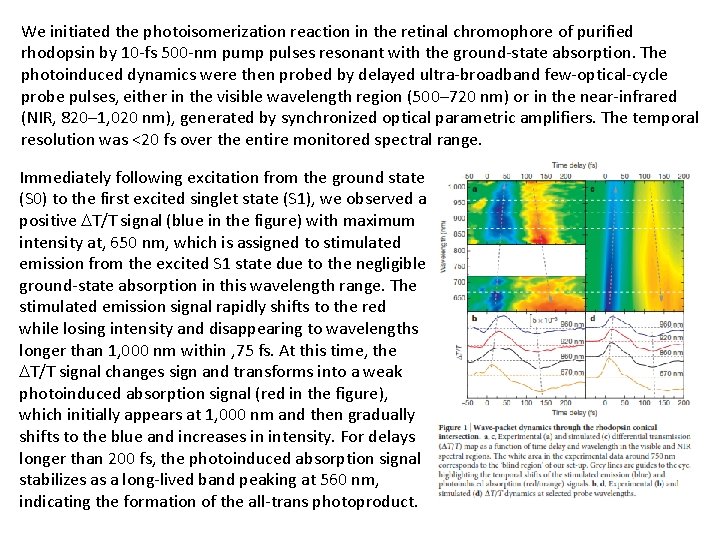
We initiated the photoisomerization reaction in the retinal chromophore of purified rhodopsin by 10 -fs 500 -nm pump pulses resonant with the ground-state absorption. The photoinduced dynamics were then probed by delayed ultra-broadband few-optical-cycle probe pulses, either in the visible wavelength region (500– 720 nm) or in the near-infrared (NIR, 820– 1, 020 nm), generated by synchronized optical parametric amplifiers. The temporal resolution was <20 fs over the entire monitored spectral range. Immediately following excitation from the ground state (S 0) to the first excited singlet state (S 1), we observed a positive T/T signal (blue in the figure) with maximum intensity at, 650 nm, which is assigned to stimulated emission from the excited S 1 state due to the negligible ground-state absorption in this wavelength range. The stimulated emission signal rapidly shifts to the red while losing intensity and disappearing to wavelengths longer than 1, 000 nm within , 75 fs. At this time, the T/T signal changes sign and transforms into a weak photoinduced absorption signal (red in the figure), which initially appears at 1, 000 nm and then gradually shifts to the blue and increases in intensity. For delays longer than 200 fs, the photoinduced absorption signal stabilizes as a long-lived band peaking at 560 nm, indicating the formation of the all-trans photoproduct.
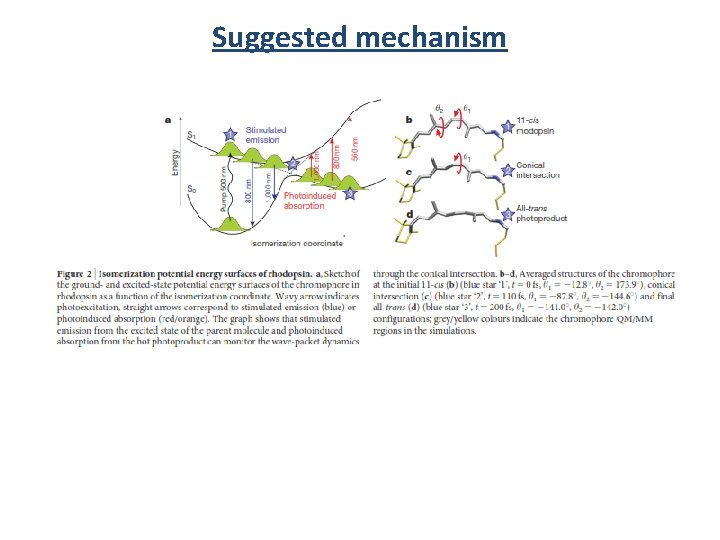
Suggested mechanism
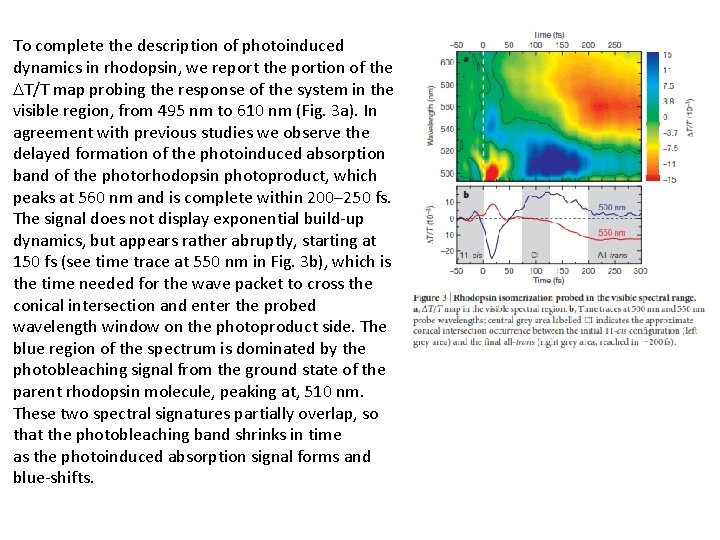
To complete the description of photoinduced dynamics in rhodopsin, we report the portion of the T/T map probing the response of the system in the visible region, from 495 nm to 610 nm (Fig. 3 a). In agreement with previous studies we observe the delayed formation of the photoinduced absorption band of the photorhodopsin photoproduct, which peaks at 560 nm and is complete within 200– 250 fs. The signal does not display exponential build-up dynamics, but appears rather abruptly, starting at 150 fs (see time trace at 550 nm in Fig. 3 b), which is the time needed for the wave packet to cross the conical intersection and enter the probed wavelength window on the photoproduct side. The blue region of the spectrum is dominated by the photobleaching signal from the ground state of the parent rhodopsin molecule, peaking at, 510 nm. These two spectral signatures partially overlap, so that the photobleaching band shrinks in time as the photoinduced absorption signal forms and blue-shifts.
- Slides: 32