Transfemoral Access Devices Tips for Closure Devices James






- Slides: 21

Transfemoral Access Devices & Tips for Closure Devices James P. Zidar, M. D. , F. A. C. C. , F. S. C. A. I Clinical Professor of Medicine, UNC Health Systems UNC Health System Physician-in-Chief, Heart & Vascular Corporate Chief of Cardiology, Rex Healthcare Raleigh, North Carolina

Disclosures • In the past year, I have been on a scientific advisory board, worked as a consultant for, or conducted clinical research for: – Abbott Vascular, BSC, Medtronic, and Siemens

Closure Devices

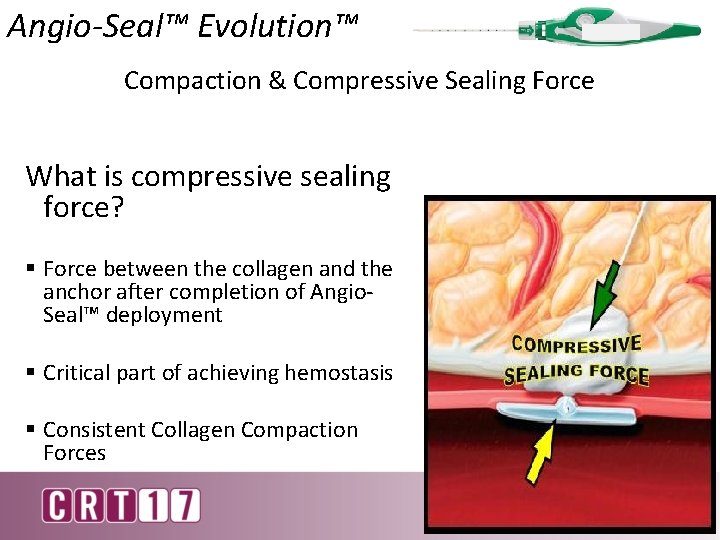
Angio-Seal™ Evolution™ Compaction & Compressive Sealing Force What is compressive sealing force? § Force between the collagen and the anchor after completion of Angio. Seal™ deployment § Critical part of achieving hemostasis § Consistent Collagen Compaction Forces

Why Big Holes? 26 Fr 23 Fr 18 Fr 14 Fr 12 Fr • Introduction of lower profile Endovascular thoracic and abdominal devices have expanded the market to a potential of 5 million people • Closure devices have facilitated the adoption of transcatheter aortic valves, AAA endografts and LV support devices for high risk PCI

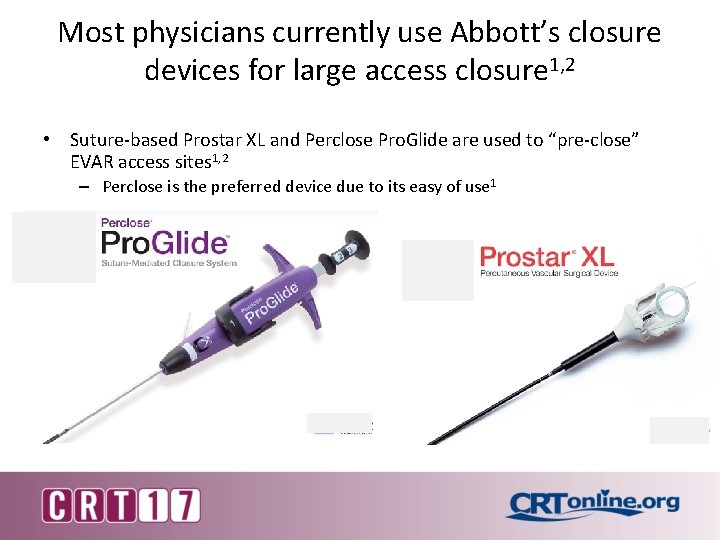
Most physicians currently use Abbott’s closure devices for large access closure 1, 2 • Suture-based Prostar XL and Perclose Pro. Glide are used to “pre-close” EVAR access sites 1, 2 – Perclose is the preferred device due to its easy of use 1

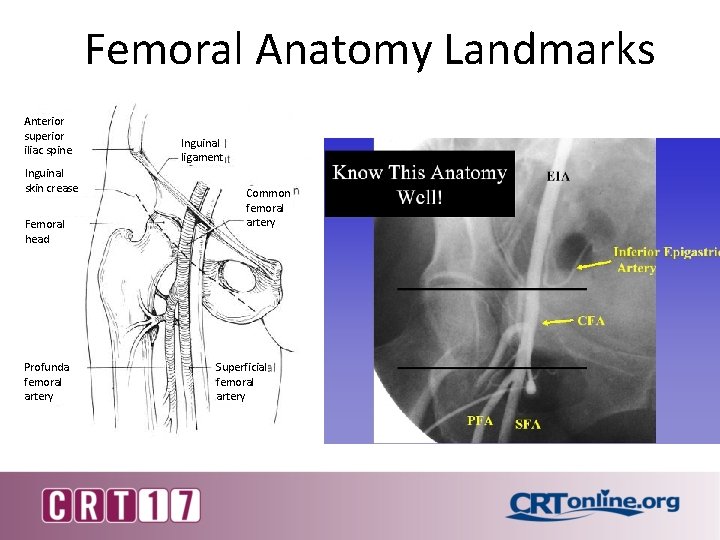
Femoral Anatomy Landmarks Anterior superior iliac spine Inguinal skin crease Femoral head Profunda femoral artery Inguinal ligament Common femoral artery Superficial femoral artery X CFA PFA SFA

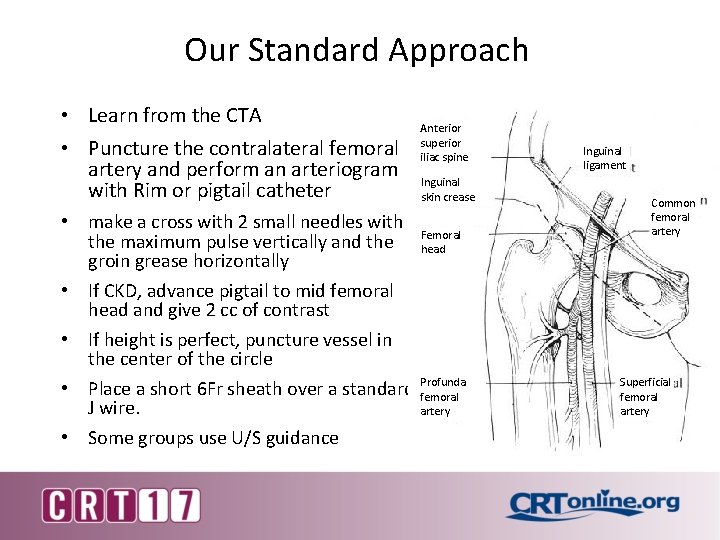
Our Standard Approach • Learn from the CTA • Puncture the contralateral femoral artery and perform an arteriogram with Rim or pigtail catheter • make a cross with 2 small needles with the maximum pulse vertically and the groin grease horizontally • If CKD, advance pigtail to mid femoral head and give 2 cc of contrast • If height is perfect, puncture vessel in the center of the circle • Place a short 6 Fr sheath over a standard J wire. • Some groups use U/S guidance Anterior superior iliac spine Inguinal skin crease Femoral head Profunda femoral artery Inguinal ligament Common femoral artery Superficial femoral artery

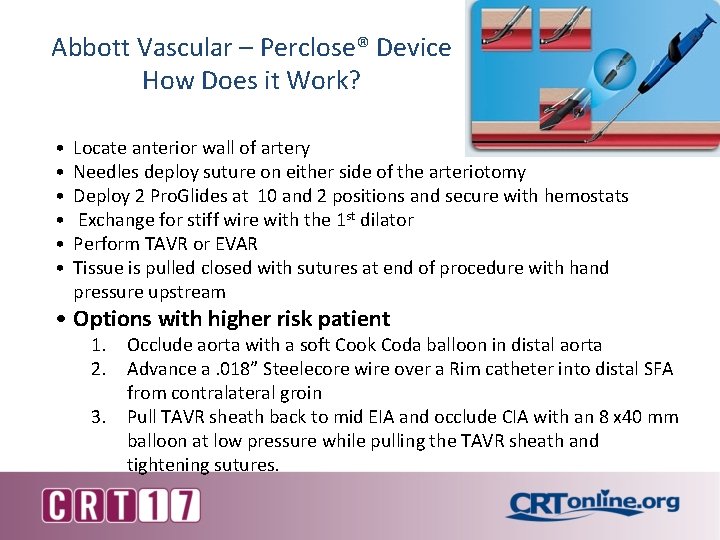
Abbott Vascular – Perclose® Device How Does it Work? • • • Locate anterior wall of artery Needles deploy suture on either side of the arteriotomy Deploy 2 Pro. Glides at 10 and 2 positions and secure with hemostats Exchange for stiff wire with the 1 st dilator Perform TAVR or EVAR Tissue is pulled closed with sutures at end of procedure with hand pressure upstream • Options with higher risk patient 1. Occlude aorta with a soft Cook Coda balloon in distal aorta 2. Advance a. 018” Steelecore wire over a Rim catheter into distal SFA from contralateral groin 3. Pull TAVR sheath back to mid EIA and occlude CIA with an 8 x 40 mm balloon at low pressure while pulling the TAVR sheath and tightening sutures.

Most physicians use suture based closure systems to “pre-close” the large arteriotomies Step 1: The device is deployed over a wire prior to any interventions 1 Step 3: Interventional devices are tracked through the vasculature with the sutures in place 1 S Step 2: The deployed device is removed leaving the sutures in place around the arteriotomy 1 Step 4: Following interventions, the vessel is closed with pre -deployed sutures 1

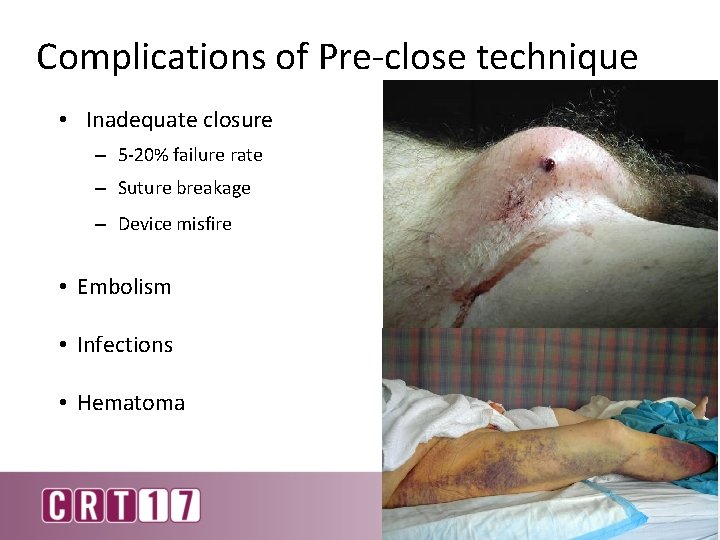
Complications of Pre-close technique • Inadequate closure – 5 -20% failure rate – Suture breakage – Device misfire • Embolism • Infections • Hematoma

• Pre-close technique: 2 Pro. Glide devices are crossed at 40 o and “pre-closed” • Each Perclose (6 Fr) device costs ~$390, which equals to ~$780/case • Prostar XL 10 approved for 8. 5 to 10 Fr closure; Each costs ~$900

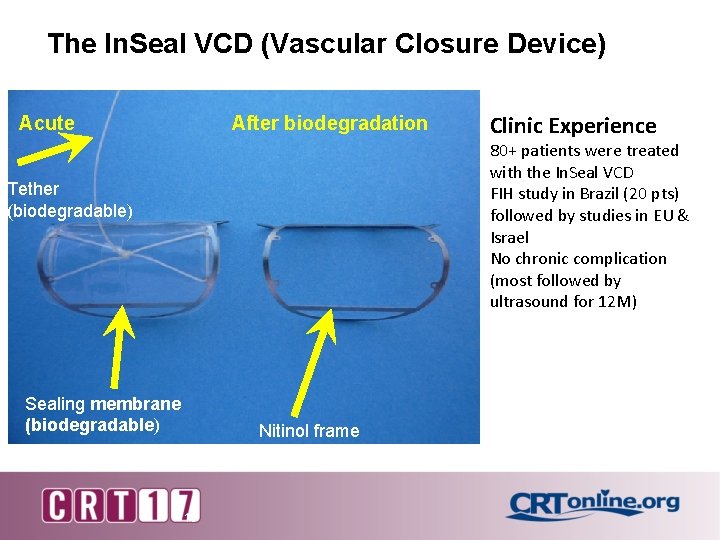
The In. Seal VCD (Vascular Closure Device) Acute After biodegradation Clinic Experience 80+ patients were treated with the In. Seal VCD FIH study in Brazil (20 pts) followed by studies in EU & Israel No chronic complication (most followed by ultrasound for 12 M) Tether (biodegradable) Sealing membrane (biodegradable) Nitinol frame 13

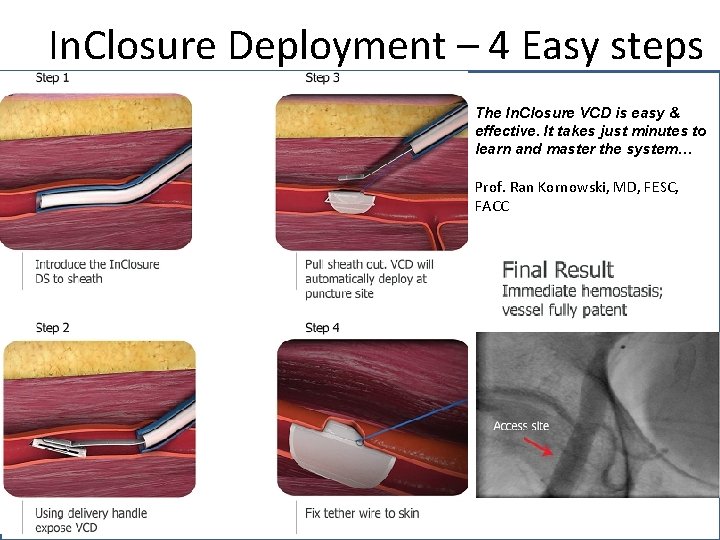
In. Closure Deployment – 4 Easy steps The In. Closure VCD is easy & effective. It takes just minutes to learn and master the system… Prof. Ran Kornowski, MD, FESC, FACC 14 In. Seal Medical Confidential

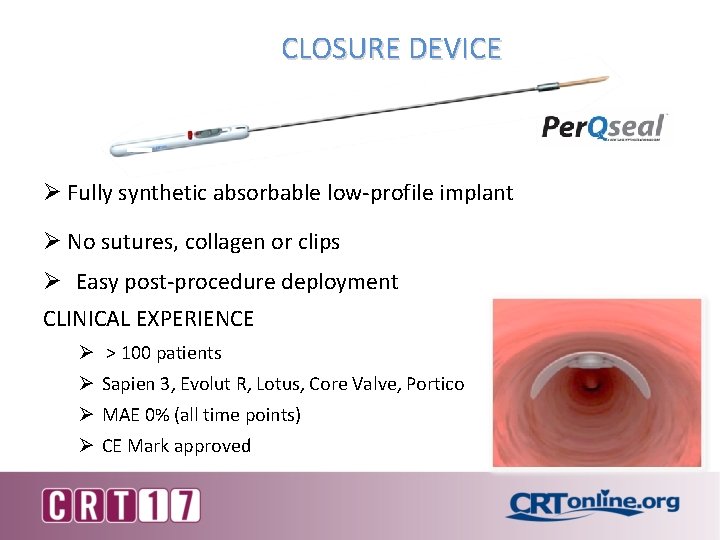
CLOSURE DEVICE Ø Fully synthetic absorbable low-profile implant Ø No sutures, collagen or clips Ø Easy post-procedure deployment CLINICAL EXPERIENCE Ø > 100 patients Ø Sapien 3, Evolut R, Lotus, Core Valve, Portico Ø MAE 0% (all time points) Ø CE Mark approved

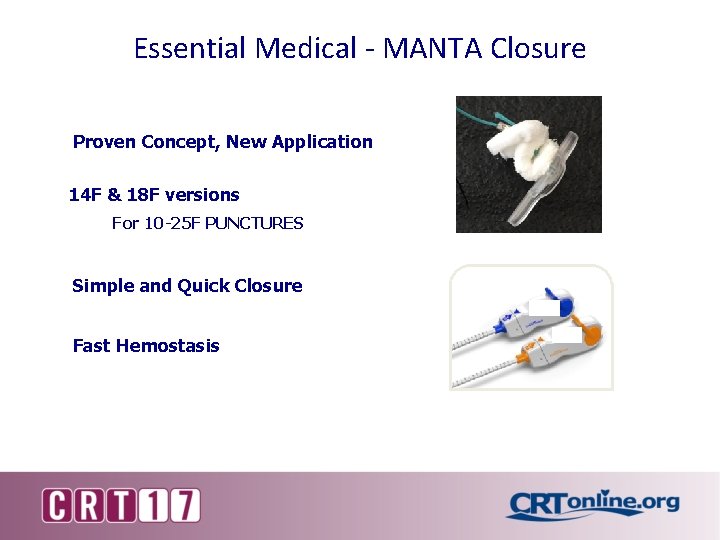
Essential Medical - MANTA Closure Proven Concept, New Application 14 F & 18 F versions For 10 -25 F PUNCTURES Simple and Quick Closure Fast Hemostasis

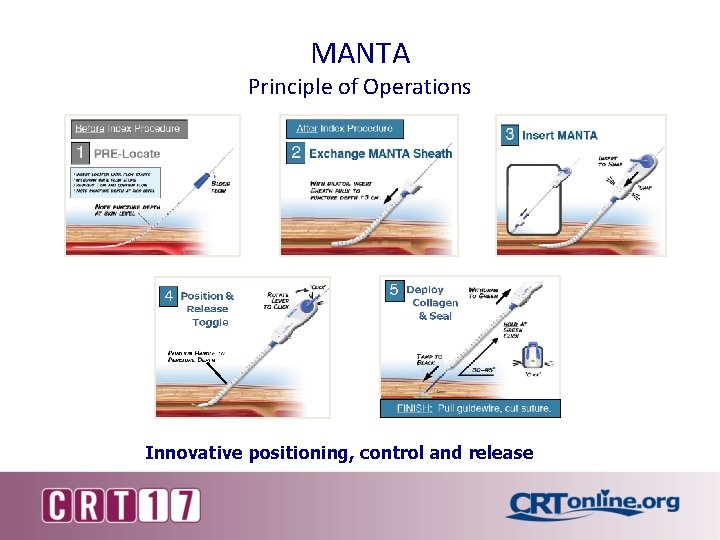
MANTA Principle of Operations Innovative positioning, control and release

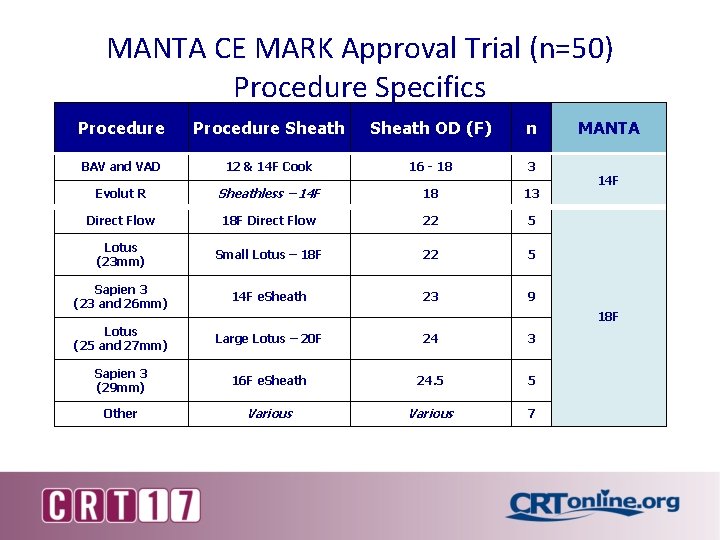
MANTA CE MARK Approval Trial (n=50) Procedure Specifics Procedure Sheath OD (F) n BAV and VAD 12 & 14 F Cook 16 - 18 3 Evolut R Sheathless – 14 F 18 13 Direct Flow 18 F Direct Flow 22 5 Lotus (23 mm) Small Lotus – 18 F 22 5 Sapien 3 (23 and 26 mm) 14 F e. Sheath 23 9 Lotus (25 and 27 mm) Large Lotus – 20 F 24 3 Sapien 3 (29 mm) 16 F e. Sheath 24. 5 5 Other Various 7 MANTA 14 F 18 F

ENDPOINTS All-cause Mortality Disabling Stroke N=50 4 (8%) 0 Minor Stroke 1 (2%) Major vascular Complications 1 (2%) covered stent Minor Vascular Complications Need for Packed Cells Transfusions 0 9 (18%) None device related Hemostasis Success 47 (94%) Hemostasis time < 10 min Deployment time 1 -2 minutes Median Time to Hemostasis 23 seconds

Vascular Closure Devices Have Patient Limitations § Peripheral vascular disease § Bifurcation sticks § Small femoral vessels § Obesity/Low BMI

Caveats • Initial Access Matters • Learning Curve • Spend the extra minute to make an adequate tissue track • We still do not have the ideal device