Transcatheter TAVR versus Surgical AVR Aortic Valve Replacement


![PARTNER cohort B (inoperable) All-Cause Mortality at 1 Year HR [95% CI] = 0. PARTNER cohort B (inoperable) All-Cause Mortality at 1 Year HR [95% CI] = 0.](https://slidetodoc.com/presentation_image_h2/c97592cd0c6c3eba11c6bd249df93dd4/image-5.jpg)
![PARTNER cohort A All-Cause Mortality at 1 Year 0. 5 HR [95% CI] = PARTNER cohort A All-Cause Mortality at 1 Year 0. 5 HR [95% CI] =](https://slidetodoc.com/presentation_image_h2/c97592cd0c6c3eba11c6bd249df93dd4/image-9.jpg)










- Slides: 51

Transcatheter (TAVR) versus Surgical (AVR) Aortic Valve Replacement: Incidence, hazard, determinants, and consequences of neurological events in the PARTNER Trial The PARTNER Stroke Substudy Writing Group* On behalf of The PARTNER Trial Investigators and Patients * Miller DC, Mack MJ, Svensson LG, Kodali SK, Kapadia S, Anderson WN, Rajeswaran J, Blackstone EH

Presenter Disclosure Information for PARTNER Trial, AATS May, 2011 D. Craig Miller , M. D. Affiliation/Financial Relationship Company Grant/ Research Support: NHLBI research grant RO 1 HL 67025 Consulting Fees/Honoraria: • The PARTNER U. S. Pivotal Trial Executive Committee, Edwards Lifesciences (uncompensated) • Stanford PI – The PARTNER Trial, Edwards Lifesciences (uncompensated) • Consultant, Abbott Vascular (Mitra. Clip) • Consultant, Medtronic Cardio. Vascular Division • Consultant, St. Jude Medical Major Stock Shareholder/Equity Interest: Royalty Income: Ownership/Founder: Salary: Intellectual Property Rights: Other Financial Benefit:

Background § Surgical AVR is the standard of care for symptomatic aortic stenosis § Survival after TAVR is superior compared to medical therapy in inoperable patients, and is non -inferior to that after AVR in high-risk operative candidates, but neurological complications occur more frequently after TAVR § No randomized trial comparing TAVR and AVR focusing on neurological events has been performed

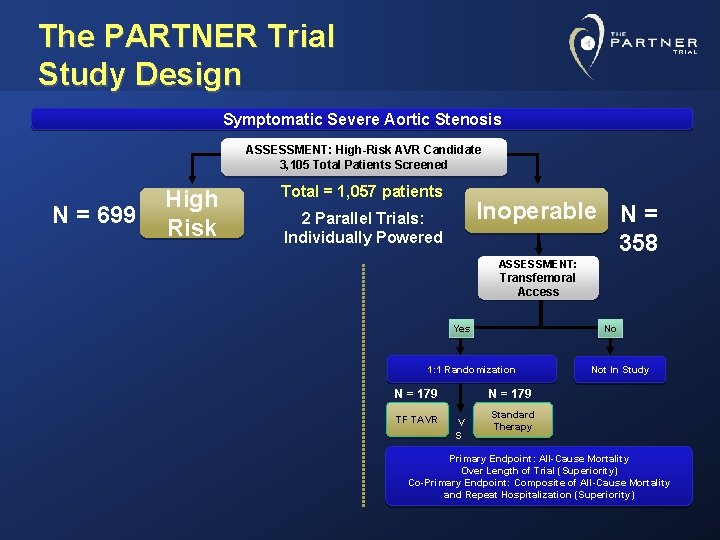
The PARTNER Trial Study Design Symptomatic Severe Aortic Stenosis ASSESSMENT: High-Risk AVR Candidate 3, 105 Total Patients Screened N = 699 High Risk Total = 1, 057 patients Inoperable N = 358 2 Parallel Trials: Individually Powered ASSESSMENT: Transfemoral Access Yes No 1: 1 Randomization N = 179 TF TAVR Not In Study N = 179 V S Standard Therapy Primary Endpoint: All-Cause Mortality Over Length of Trial (Superiority) Co-Primary Endpoint: Composite of All-Cause Mortality and Repeat Hospitalization (Superiority)
![PARTNER cohort B inoperable AllCause Mortality at 1 Year HR 95 CI 0 PARTNER cohort B (inoperable) All-Cause Mortality at 1 Year HR [95% CI] = 0.](https://slidetodoc.com/presentation_image_h2/c97592cd0c6c3eba11c6bd249df93dd4/image-5.jpg)
PARTNER cohort B (inoperable) All-Cause Mortality at 1 Year HR [95% CI] = 0. 54 [0. 38, 0. 78] P (log rank) < 0. 0001 ∆ at 1 yr = 20. 0% NNT = 5. 0 pts Standard Rx All-cause mortality (%) TAVI 50. 7% 30. 7% Months Numbers at Risk TAVR 179 Standard Rx 179 138 121 122 83 67 41 26 12

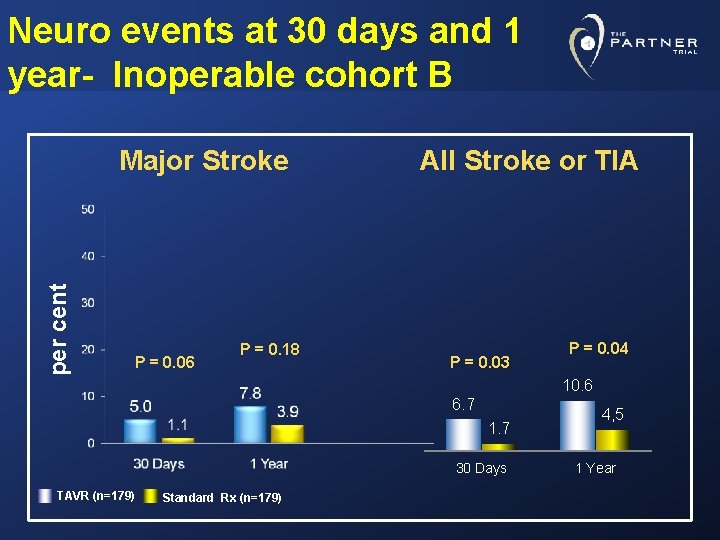
Neuro events at 30 days and 1 year- Inoperable cohort B per cent Major Stroke P = 0. 06 P = 0. 18 All Stroke or TIA P = 0. 03 P = 0. 04 10. 6 6. 7 1. 7 30 Days TAVR (n=179) Standard Rx (n=179) 4, 5 1 Year

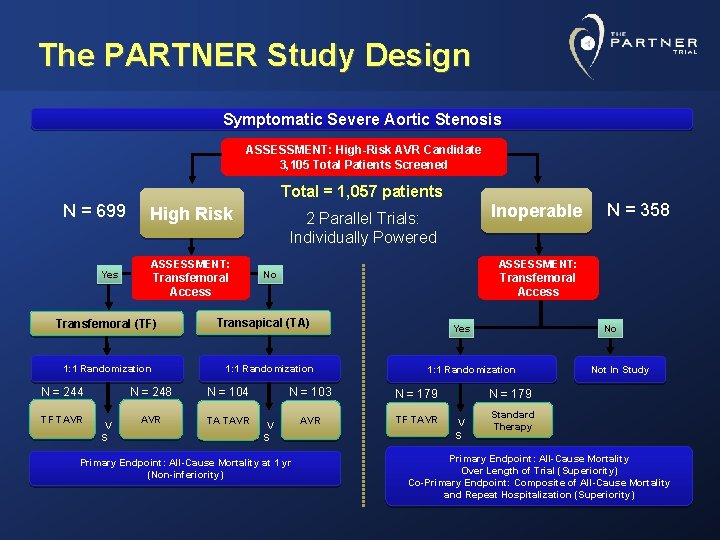
The PARTNER Study Design Symptomatic Severe Aortic Stenosis ASSESSMENT: High-Risk AVR Candidate 3, 105 Total Patients Screened Total = 1, 057 patients N = 699 Yes High Risk ASSESSMENT: Transfemoral Access Transfemoral (TF) 1: 1 Randomization N = 244 TF TAVR V S Transfemoral Access 1: 1 Randomization N = 104 AVR TA TAVR N = 358 ASSESSMENT: No Transapical (TA) N = 248 Inoperable 2 Parallel Trials: Individually Powered Yes 1: 1 Randomization N = 103 N = 179 AVR TF TAVR V S Primary Endpoint: All-Cause Mortality at 1 yr (Non-inferiority) No Not In Study N = 179 V S Standard Therapy Primary Endpoint: All-Cause Mortality Over Length of Trial (Superiority) Co-Primary Endpoint: Composite of All-Cause Mortality and Repeat Hospitalization (Superiority)

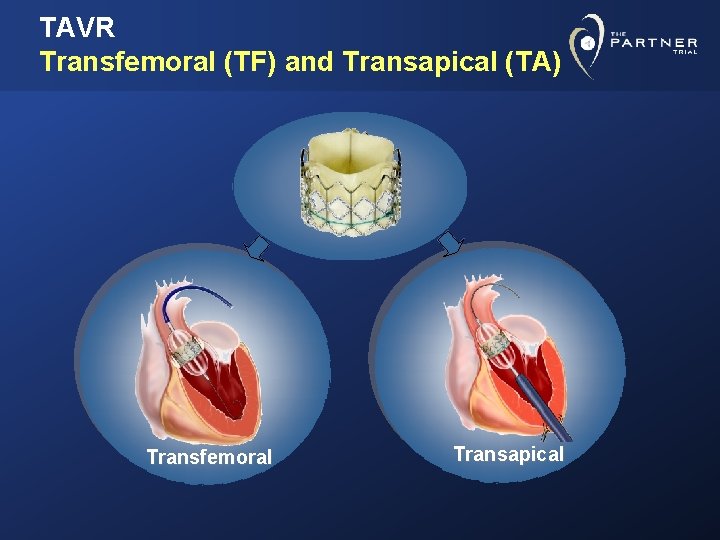
TAVR Transfemoral (TF) and Transapical (TA) Transfemoral Transapical
![PARTNER cohort A AllCause Mortality at 1 Year 0 5 HR 95 CI PARTNER cohort A All-Cause Mortality at 1 Year 0. 5 HR [95% CI] =](https://slidetodoc.com/presentation_image_h2/c97592cd0c6c3eba11c6bd249df93dd4/image-9.jpg)
PARTNER cohort A All-Cause Mortality at 1 Year 0. 5 HR [95% CI] = 0. 93 [0. 71, 1. 22] P (log rank) = 0. 62 26. 8 TAVR 0. 4 0. 3 0. 2 24. 2 0. 1 0 0 6 No. at Risk 12 18 24 Months TAVR 348 298 260 147 67 AVR 351 252 236 139 65

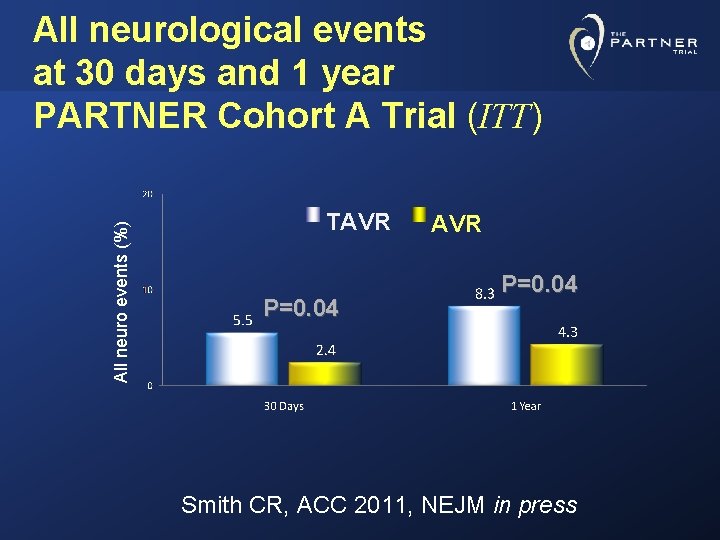
All neuro events (%) All neurological events at 30 days and 1 year PARTNER Cohort A Trial (ITT) TAVR P=0. 04 Smith CR, ACC 2011, NEJM in press

Purpose • Analyze stroke and TIA after TAVR and surgical AVR in high-risk (≈15%, floor= STS 8 -9%), operable patients with symptomatic, severe aortic stenosis in the PARTNER Trial • “As Treated” (AT) patients n= 657 (vs. ITT) • Captured all neurological events at all times • Prospective, independent, blinded adjudication of adverse neurological events by CEC, supplemented by CEC retrospective assessment of stroke severity • Unblinded re-review of all CEC summaries and source documents by 2 investigators (DCM, MJM)

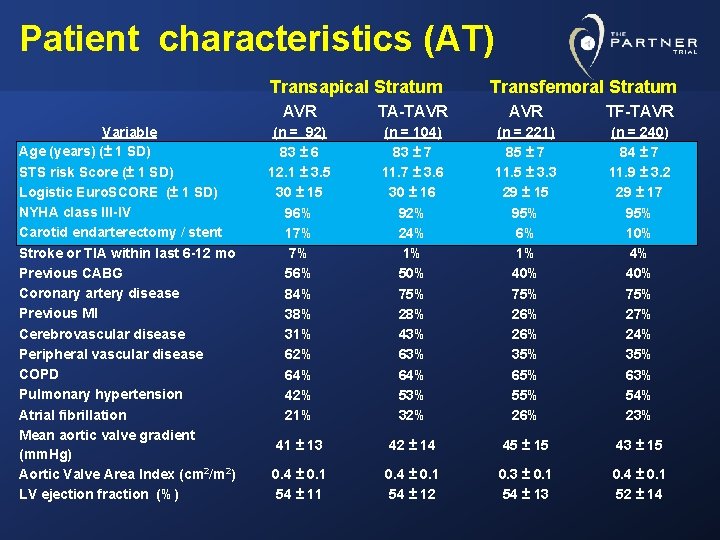
Patient characteristics (AT) Transapical Stratum Variable Age (years) (± 1 SD) STS risk Score (± 1 SD) Logistic Euro. SCORE (± 1 SD) NYHA class III-IV Carotid endarterectomy / stent Stroke or TIA within last 6 -12 mo Previous CABG Coronary artery disease Previous MI Cerebrovascular disease Peripheral vascular disease COPD Pulmonary hypertension Atrial fibrillation Mean aortic valve gradient (mm. Hg) Aortic Valve Area Index (cm 2/m 2) LV ejection fraction (%) Transfemoral Stratum AVR TA-TAVR TF-TAVR (n = 92) 83 ± 6 12. 1 ± 3. 5 30 ± 15 96% 17% 7% 56% 84% 38% 31% 62% 64% 42% 21% (n = 104) 83 ± 7 11. 7 ± 3. 6 30 ± 16 92% 24% 1% 50% 75% 28% 43% 64% 53% 32% (n = 221) 85 ± 7 11. 5 ± 3. 3 29 ± 15 95% 6% 1% 40% 75% 26% 35% 65% 55% 26% (n = 240) 84 ± 7 11. 9 ± 3. 2 29 ± 17 95% 10% 4% 40% 75% 27% 24% 35% 63% 54% 23% 41 ± 13 42 ± 14 45 ± 15 43 ± 15 0. 4 ± 0. 1 54 ± 11 0. 4 ± 0. 1 54 ± 12 0. 3 ± 0. 1 54 ± 13 0. 4 ± 0. 1 52 ± 14

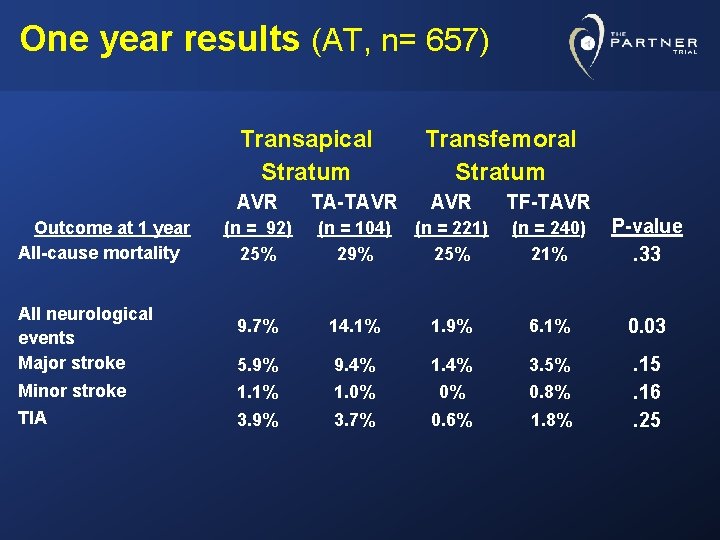
One year results (AT, n= 657) Transapical Stratum Transfemoral Stratum AVR TA-TAVR TF-TAVR (n = 92) 25% (n = 104) 29% (n = 221) 25% (n = 240) 21% P-value. 33 9. 7% 14. 1% 1. 9% 6. 1% 0. 03 5. 9% 9. 4% 1. 4% 3. 5% Minor stroke 1. 1% 1. 0% 0% 0. 8% TIA 3. 9% 3. 7% 0. 6% 1. 8% . 15. 16. 25 Outcome at 1 year All-cause mortality All neurological events Major stroke

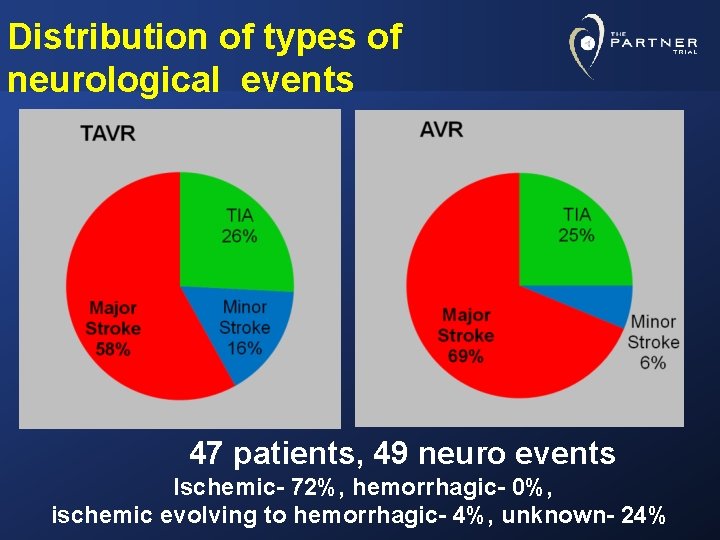
Distribution of types of neurological events 47 patients, 49 neuro events Ischemic- 72%, hemorrhagic- 0%, ischemic evolving to hemorrhagic- 4%, unknown- 24%

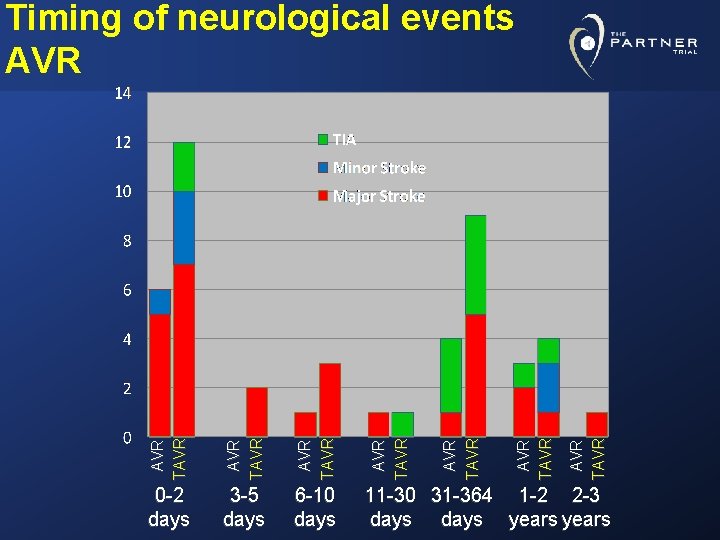
AVR TAVR 6 -10 days AVR TAVR 3 -5 days AVR TAVR 0 -2 days AVR TAVR Timing of neurological events AVR 11 -30 31 -364 1 -2 2 -3 days years

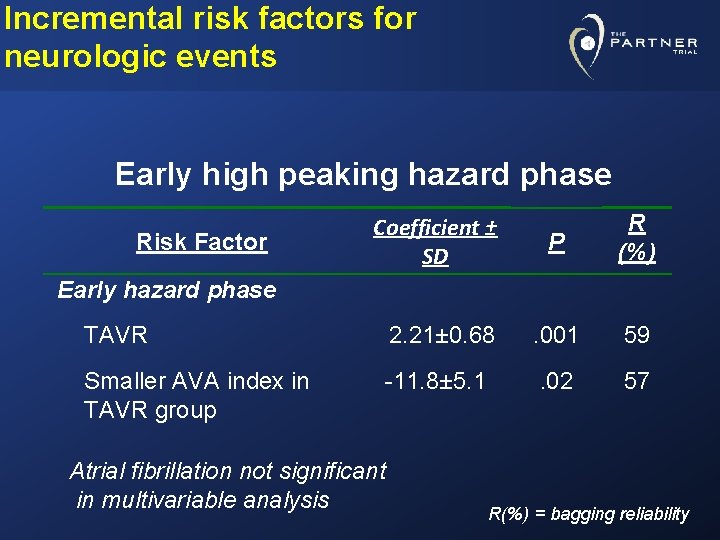
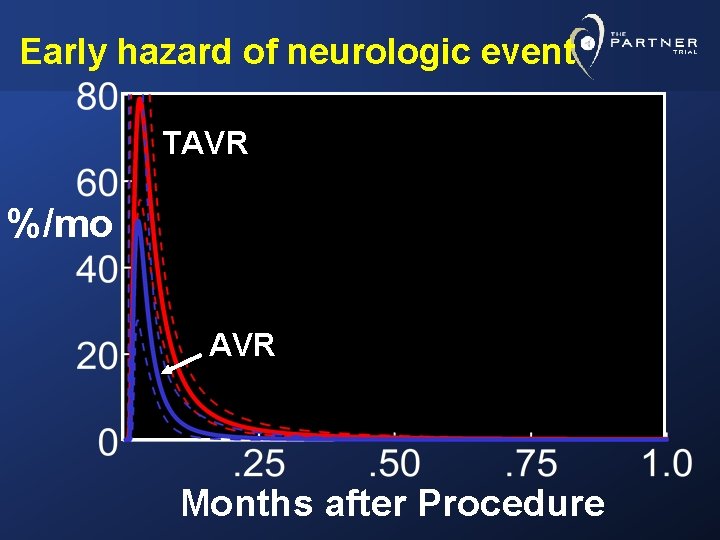
Risk Factors for Neurologic Events Multiphase, multivariable non-proportional hazard analysis Ø Early high peaking hazard phase Ø Later constant hazard phase

Incremental risk factors for neurologic events Early high peaking hazard phase Coefficient ± SD P R (%) TAVR 2. 21± 0. 68 . 001 59 Smaller AVA index in TAVR group -11. 8± 5. 1 . 02 57 Risk Factor Early hazard phase Atrial fibrillation not significant in multivariable analysis R(%) = bagging reliability

Early hazard of neurologic event TAVR %/mo AVR Months after Procedure

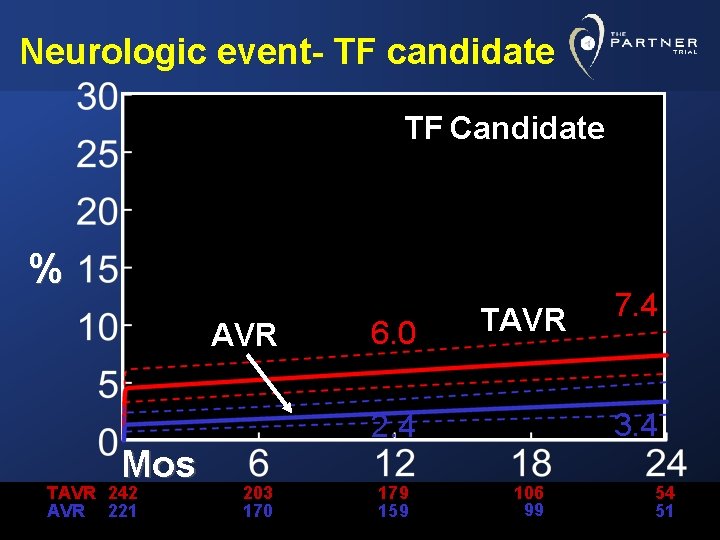
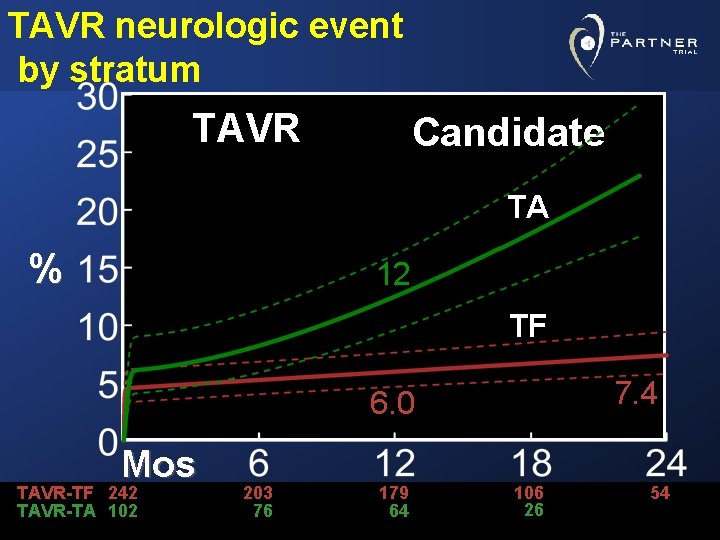
Neurologic event- TF candidate TF Candidate % AVR Mos TAVR 242 AVR 221 6. 0 TAVR 3. 4 203 170 179 159 7. 4 106 99 54 51

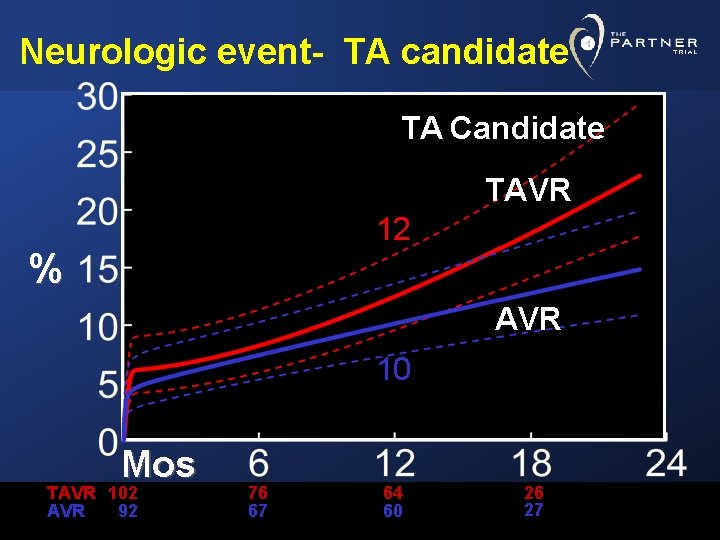
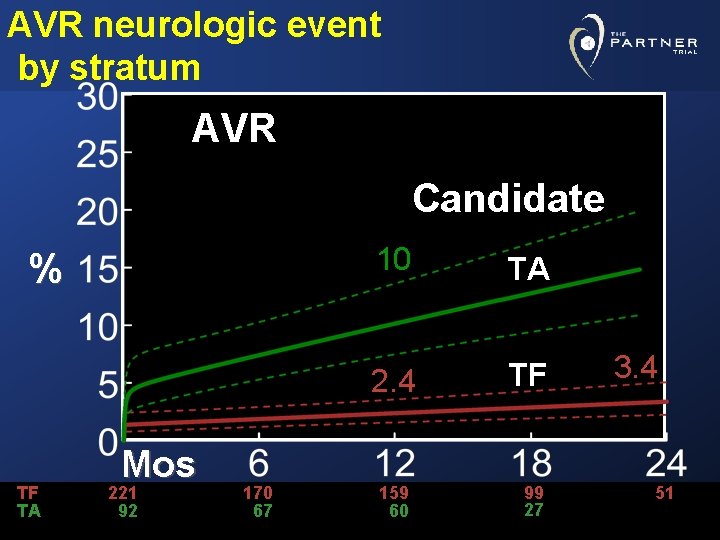
Neurologic event- TA candidate TA Candidate TAVR 12 % AVR 10 Mos TAVR 102 AVR 92 76 67 64 60 26 27

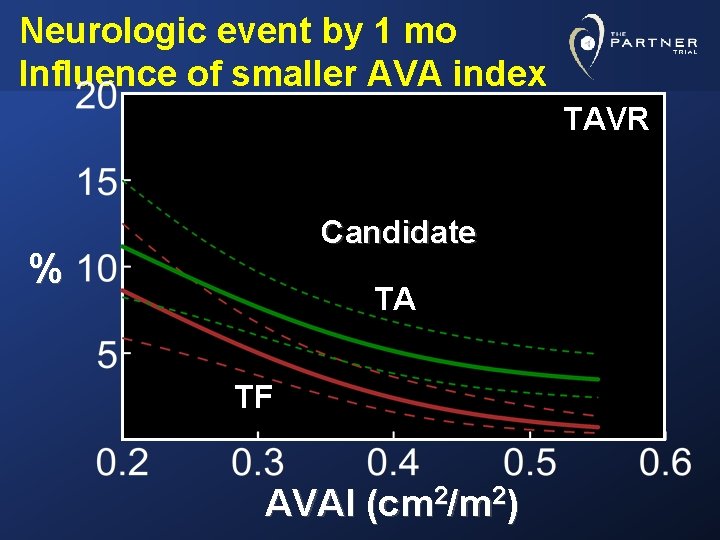
Neurologic event by 1 mo Influence of smaller AVA index TAVR Candidate % TA TF AVAI (cm 2/m 2)

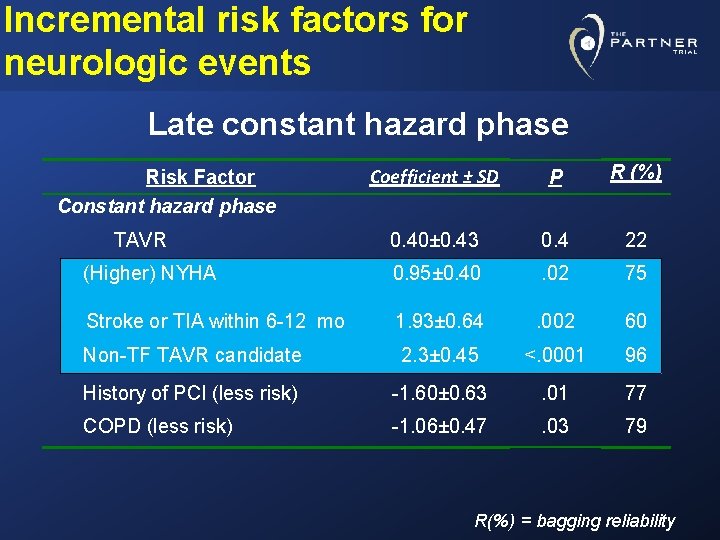
Incremental risk factors for neurologic events Late constant hazard phase Coefficient ± SD P R (%) 0. 40± 0. 43 0. 4 22 (Higher) NYHA 0. 95± 0. 40 . 02 75 Stroke or TIA within 6 -12 mo 1. 93± 0. 64 . 002 60 Non-TF TAVR candidate 2. 3± 0. 45 <. 0001 96 History of PCI (less risk) -1. 60± 0. 63 . 01 77 COPD (less risk) -1. 06± 0. 47 . 03 79 Risk Factor Constant hazard phase TAVR R(%) = bagging reliability

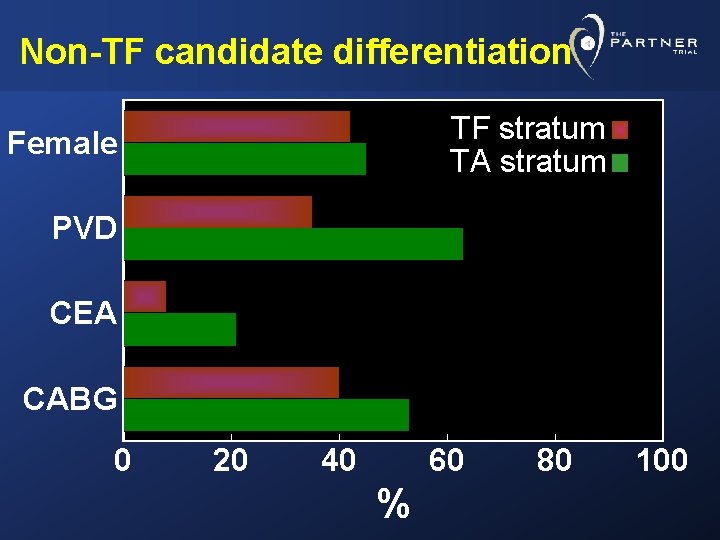
Non-TF candidate differentiation TF stratum TA stratum Female PVD CEA CABG 0 20 40 60 % 80 100

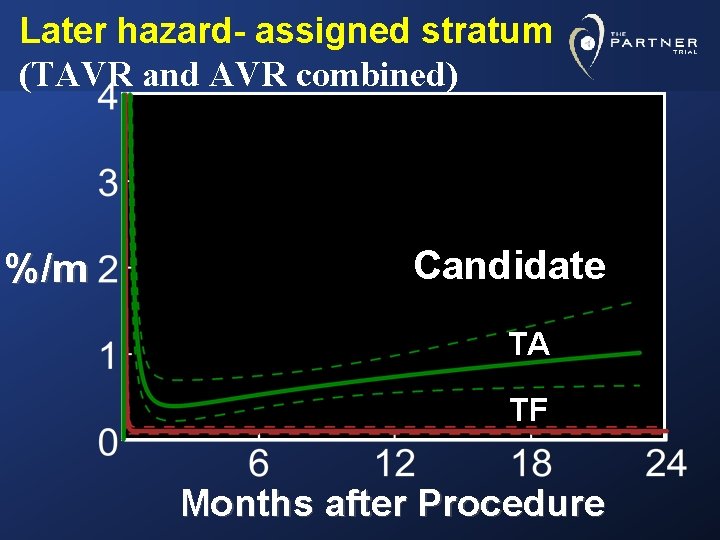
Later hazard- assigned stratum (TAVR and AVR combined) %/m Candidate TA TF Months after Procedure

TAVR neurologic event by stratum TAVR Candidate TA % 12 TF 7. 4 6. 0 Mos TAVR-TF 242 TAVR-TA 102 203 76 179 64 106 26 54

AVR neurologic event by stratum AVR Candidate % TF TA Mos 221 92 170 67 10 TA 2. 4 TF 159 60 99 27 3. 4 51

Major Stroke Small number of events n= 29 Conservative definition (modified Rankin score ≥ 2) If stroke severity unclear, categorized as major

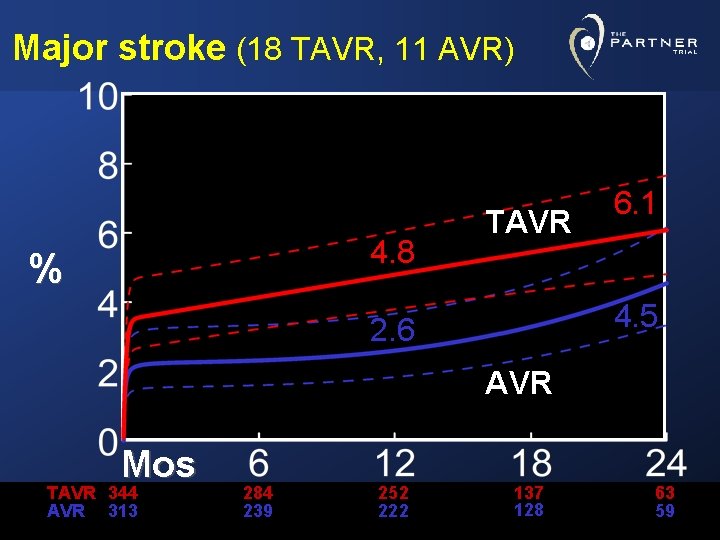
Major stroke (18 TAVR, 11 AVR) 4. 8 % TAVR 6. 1 4. 5 2. 6 AVR Mos TAVR 344 AVR 313 284 239 252 222 137 128 63 59

Competing Risks of Death and Neurologic Events

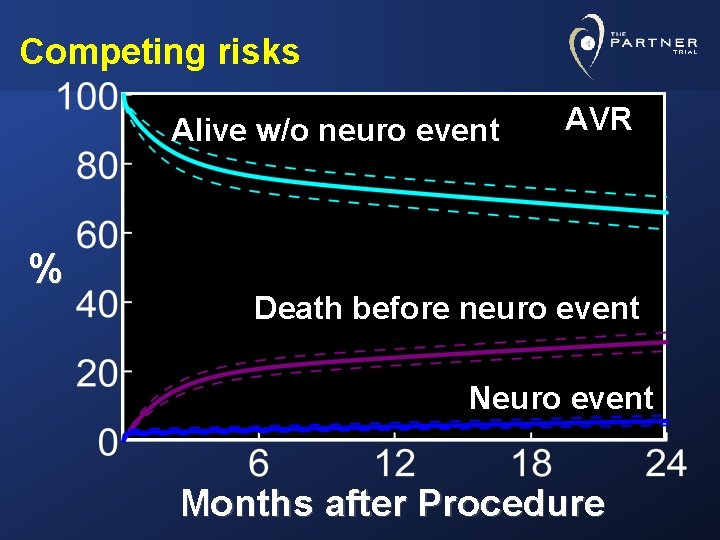
Competing risks Alive w/o neuro event % AVR Death before neuro event Neuro event Months after Procedure

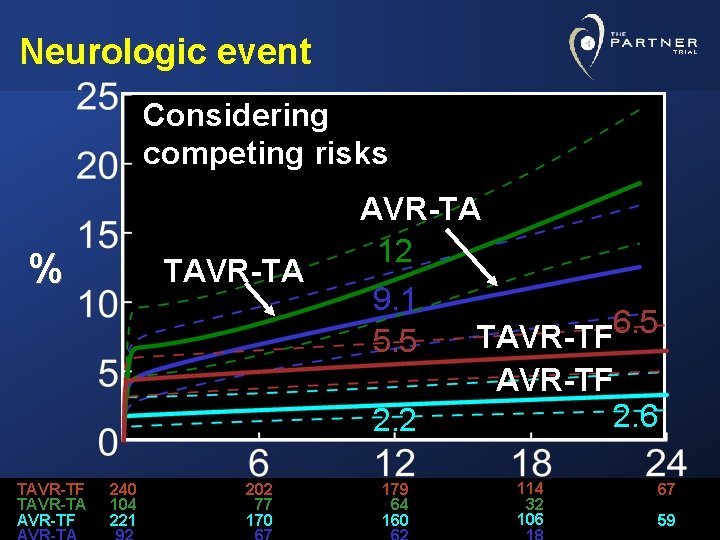
Neurologic event Considering competing risks % TAVR-TF TAVR-TA 240 104 221 202 77 170 AVR-TA 12 9. 1 6. 5 TAVR-TF 5. 5 AVR-TF 2. 6 2. 2 179 64 160 114 32 106 67 59

“Mortality Cost” of a Neurologic Event

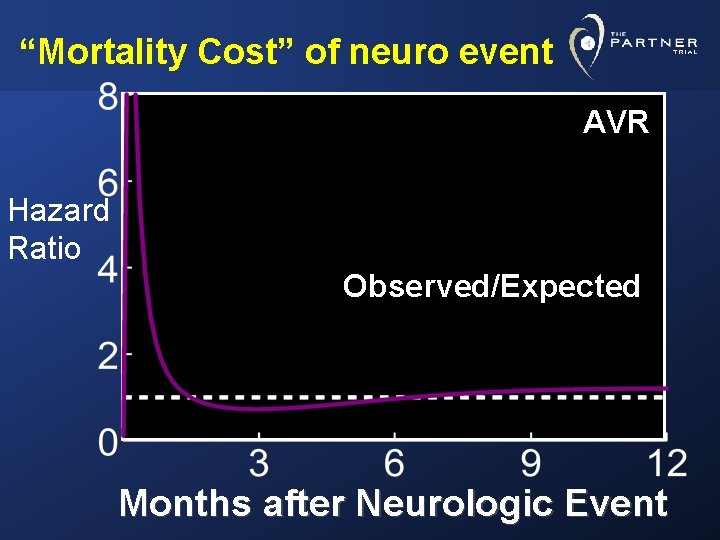
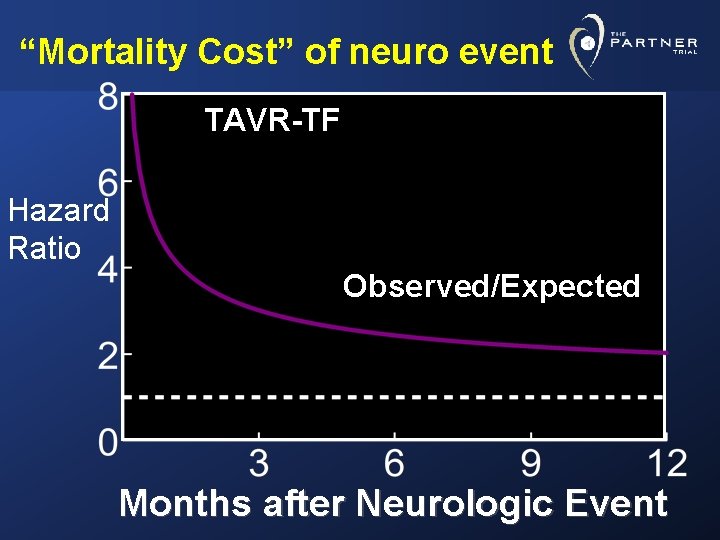
“Mortality Cost” of neuro event AVR Hazard Ratio Observed/Expected Months after Neurologic Event

“Mortality Cost” of neuro event TAVR-TF Hazard Ratio Observed/Expected Months after Neurologic Event

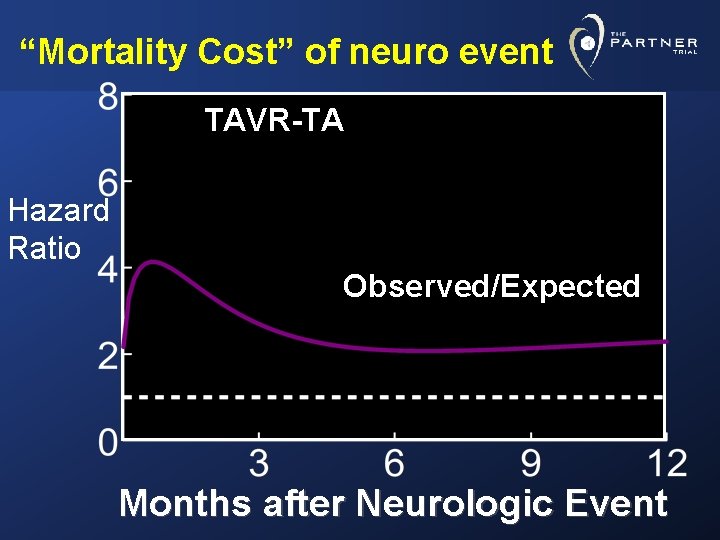
“Mortality Cost” of neuro event TAVR-TA Hazard Ratio Observed/Expected Months after Neurologic Event

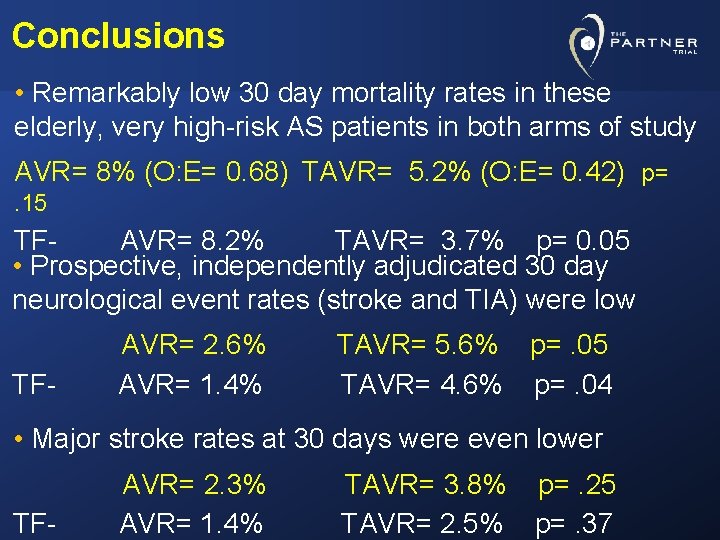
Conclusions • Remarkably low 30 day mortality rates in these elderly, very high-risk AS patients in both arms of study AVR= 8% (O: E= 0. 68) TAVR= 5. 2% (O: E= 0. 42) p=. 15 TFAVR= 8. 2% TAVR= 3. 7% p= 0. 05 • Prospective, independently adjudicated 30 day neurological event rates (stroke and TIA) were low TF- AVR= 2. 6% AVR= 1. 4% TAVR= 5. 6% p=. 05 TAVR= 4. 6% p=. 04 • Major stroke rates at 30 days were even lower TF- AVR= 2. 3% AVR= 1. 4% TAVR= 3. 8% p=. 25 TAVR= 2. 5% p=. 37

Conclusions Incremental risk factors for neurological events • Early peaking high hazard phase: TAVR Smaller AVA index (TAVR group only) • Later constant hazard phase: Generalized heavy arteriosclerotic burden (“non-TF TAVR candidate”) Stroke/TIA within 6 -12 months Higher NYHA class

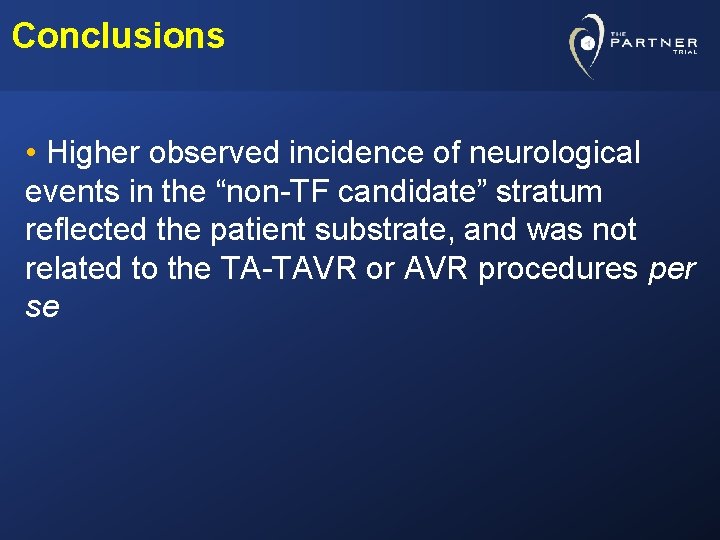
Conclusions • Higher observed incidence of neurological events in the “non-TF candidate” stratum reflected the patient substrate, and was not related to the TA-TAVR or AVR procedures per se

Conclusions • Taking competing hazard of death into consideration, the likelihood of a neurologic event was lowest in AVR patients and highest in TA-TAVR group • A neurologic event raised the risk of mortality • In AVR group: High peak, quickly returning to baseline hazard • In TAVR groups: After initial peak, risk remained elevated throughout the 24 months of follow-up, particularly in TA stratum

Limitations • These results can only be interpreted within the constraints of the PARTNER Trial protocol: • Carefully controlled patient selection • Regimented training and proctoring • Critical case monitoring and review • Dedicated multi-disciplinary “Heart Valve Team” in these 26 centers • “TF first” protocol philosophy and TAVR sheath sizes available • Learning curve, first generation TAVR device • Not adequately powered for TF vs. TA comparison

Thank You

BACK-UP

Inferences Can TAVR stroke rate be lowered? EARLY HIGH HAZARD PHASE • Peri-procedural anticoagulation management • Clopidogrel load, + dual antiplatelet Rx • Warfarin or dabigatran Rx • No protamine reversal (TF) • Bridge AF patients with heparin • Cerebral embolic prevention devices • Newer low profile THV deployment systems • Carotid compression during BAV, THV deployment LATE CONSTANT HAZARD PHASE • More rigorous patient selection (TA)

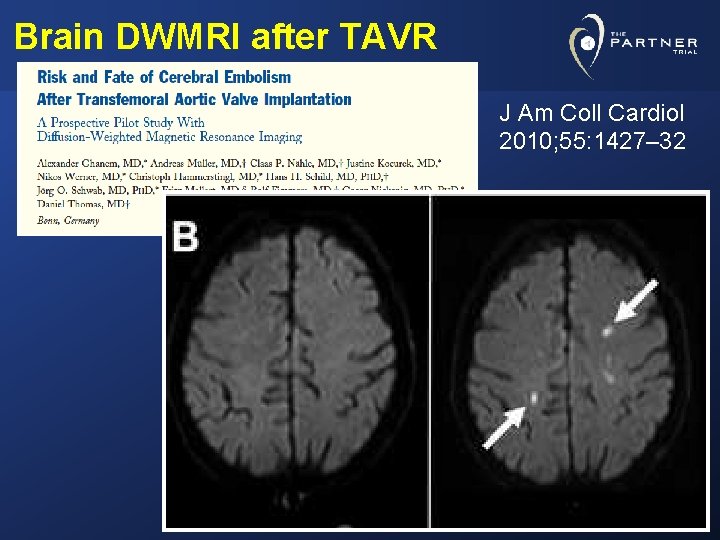
Brain DWMRI after TAVR J Am Coll Cardiol 2010; 55: 1427– 32

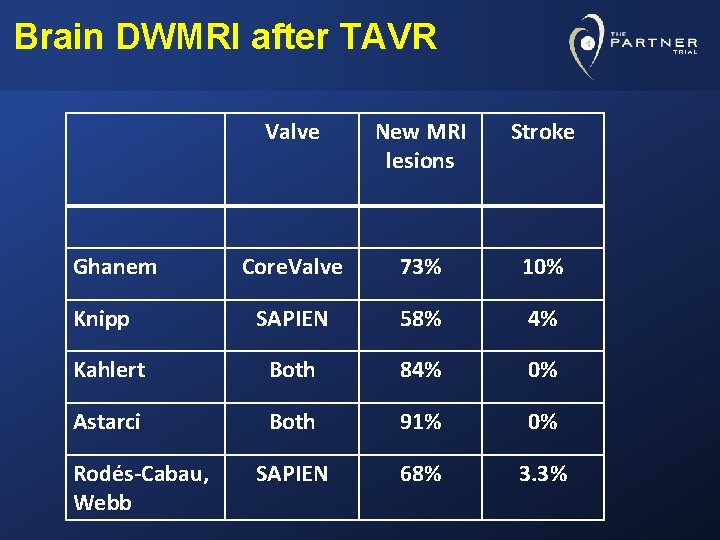
Brain DWMRI after TAVR Valve New MRI lesions Stroke Core. Valve 73% 10% SAPIEN 58% 4% Kahlert Both 84% 0% Astarci Both 91% 0% SAPIEN 68% 3. 3% Ghanem Knipp Rodés-Cabau, Webb

Embrella® Embolic Deflector Initial Vancouver experience in 4 patients, 3 with TAVI and 1 with BAV Effectiveness? Safety? Nietlispach et al. , J Am Coll Cardiol Intv 2010; 3: 1133– 8

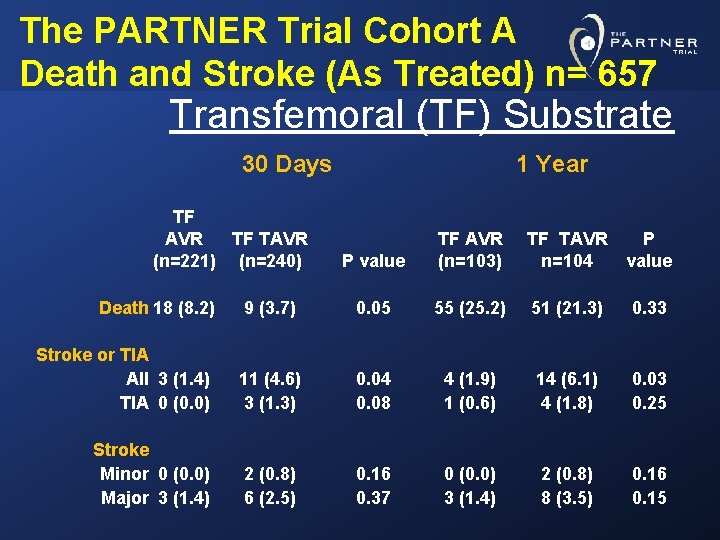
The PARTNER Trial Cohort A Death and Stroke (As Treated) n= 657 Transfemoral (TF) Substrate 30 Days TF AVR TF TAVR (n=221) (n=240) 1 Year P value TF AVR (n=103) TF TAVR n=104 P value Death 18 (8. 2) 9 (3. 7) 0. 05 55 (25. 2) 51 (21. 3) 0. 33 Stroke or TIA All 3 (1. 4) TIA 0 (0. 0) 11 (4. 6) 3 (1. 3) 0. 04 0. 08 4 (1. 9) 1 (0. 6) 14 (6. 1) 4 (1. 8) 0. 03 0. 25 Stroke Minor 0 (0. 0) Major 3 (1. 4) 2 (0. 8) 6 (2. 5) 0. 16 0. 37 0 (0. 0) 3 (1. 4) 2 (0. 8) 8 (3. 5) 0. 16 0. 15

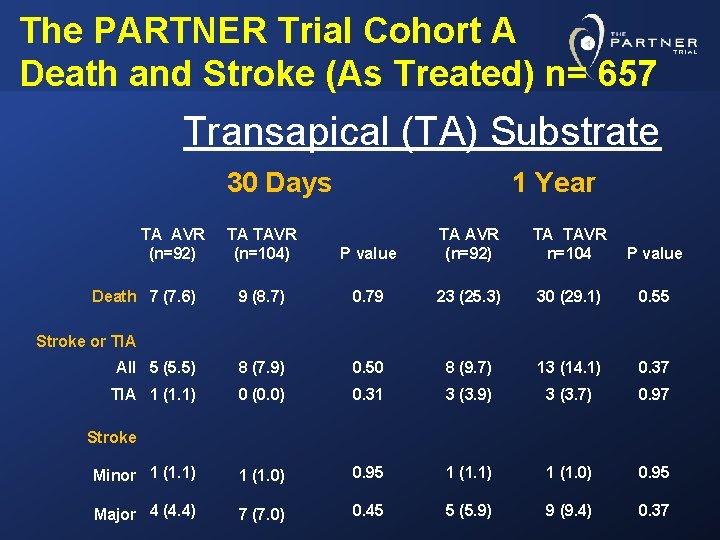
The PARTNER Trial Cohort A Death and Stroke (As Treated) n= 657 Transapical (TA) Substrate 30 Days TA AVR (n=92) P value TA AVR (n=92) TA TAVR n=104 P value 9 (8. 7) 0. 79 23 (25. 3) 30 (29. 1) 0. 55 All 5 (5. 5) 8 (7. 9) 0. 50 8 (9. 7) 13 (14. 1) 0. 37 TIA 1 (1. 1) 0 (0. 0) 0. 31 3 (3. 9) 3 (3. 7) 0. 97 Minor 1 (1. 1) 1 (1. 0) 0. 95 Major 4 (4. 4) 7 (7. 0) 0. 45 5 (5. 9) 9 (9. 4) 0. 37 Death 7 (7. 6) TA TAVR (n=104) 1 Year Stroke or TIA Stroke

Stroke Definition- The Modified Rankin Scale Minor • 0 - No Symptoms • 1 - No significant disability. Able to carry out all usual activities, despite some symptoms Major • 2 - Slight disability. Able to look after own affairs without assistance, but unable to carry out all previous activities. • 3 - Moderate disability. Requires some help, but able to walk unassisted. • 4 - Moderately severe disability. Unable to attend to own bodily needs without assistance, and unable to walk unassisted. • 5 - Severe disability. Requires constant nursing care and attention, bedridden, incontinent. • 6 - Dead.

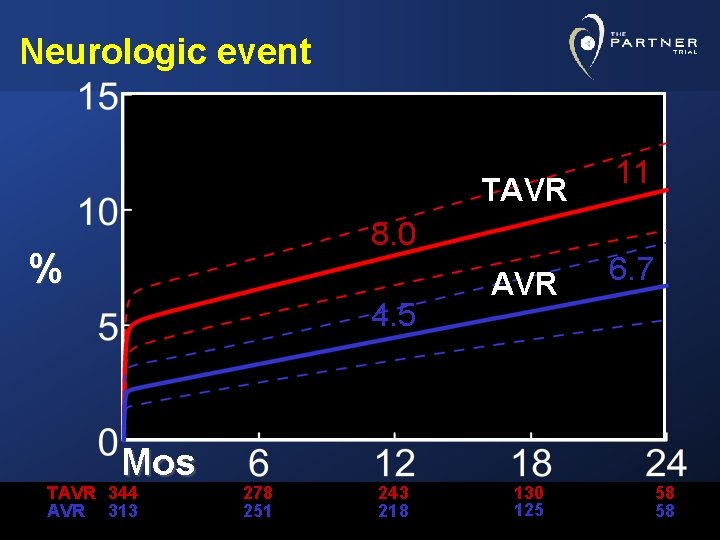
Neurologic event TAVR 8. 0 % 4. 5 Mos TAVR 344 AVR 313 278 251 243 218 AVR 130 125 11 6. 7 58 58

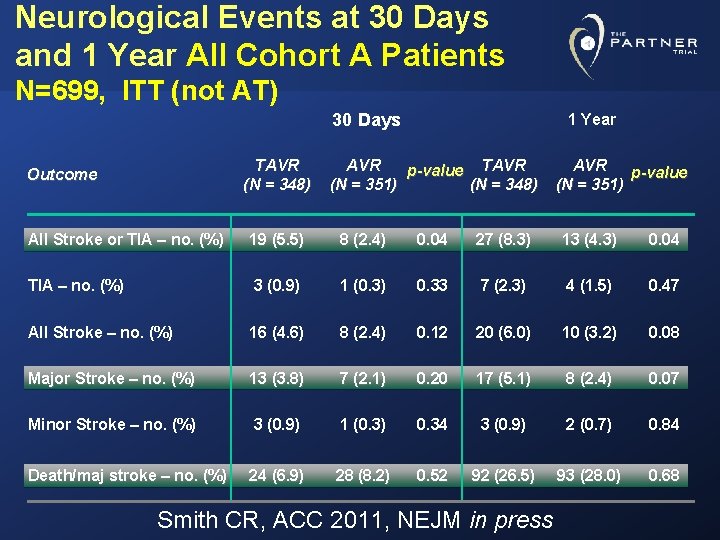
Neurological Events at 30 Days and 1 Year All Cohort A Patients N=699, ITT (not AT) 30 Days TAVR (N = 348) Outcome 1 Year AVR p-value TAVR (N = 351) (N = 348) AVR p-value (N = 351) All Stroke or TIA – no. (%) 19 (5. 5) 8 (2. 4) 0. 04 27 (8. 3) 13 (4. 3) 0. 04 TIA – no. (%) 3 (0. 9) 1 (0. 3) 0. 33 7 (2. 3) 4 (1. 5) 0. 47 All Stroke – no. (%) 16 (4. 6) 8 (2. 4) 0. 12 20 (6. 0) 10 (3. 2) 0. 08 Major Stroke – no. (%) 13 (3. 8) 7 (2. 1) 0. 20 17 (5. 1) 8 (2. 4) 0. 07 Minor Stroke – no. (%) 3 (0. 9) 1 (0. 3) 0. 34 3 (0. 9) 2 (0. 7) 0. 84 Death/maj stroke – no. (%) 24 (6. 9) 28 (8. 2) 0. 52 92 (26. 5) 93 (28. 0) 0. 68 Smith CR, ACC 2011, NEJM in press