Transcatheter LAA Occlusion Ahmed A Khattab Cardiology Bern

Transcatheter LAA Occlusion Ahmed A. Khattab Cardiology Bern – Switzerland
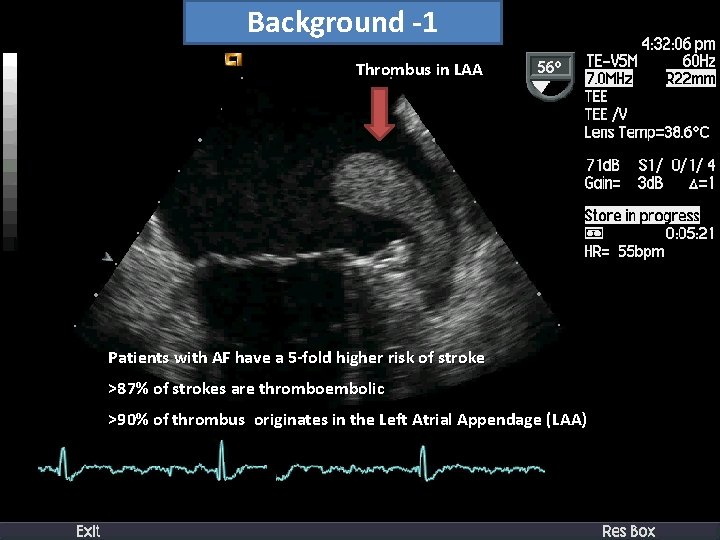
Background -1 Thrombus in LAA Patients with AF have a 5 -fold higher risk of stroke >87% of strokes are thromboembolic >90% of thrombus originates in the Left Atrial Appendage (LAA)

Background -2 • Simultaneous surgical closure during cardiac surgery has been common practice since many years and is recommended in current guidelines. • Thoracoscopic epicardial occlusion under general anaesthesia is an option. • Non-surgical transcatheter LAA exclusion was first introduced in 2001. Bonow RO, et al. JACC, 2006. Blackshear JL, et al. JACC, 2003. Sievert H, et al. Circulation, 2002.
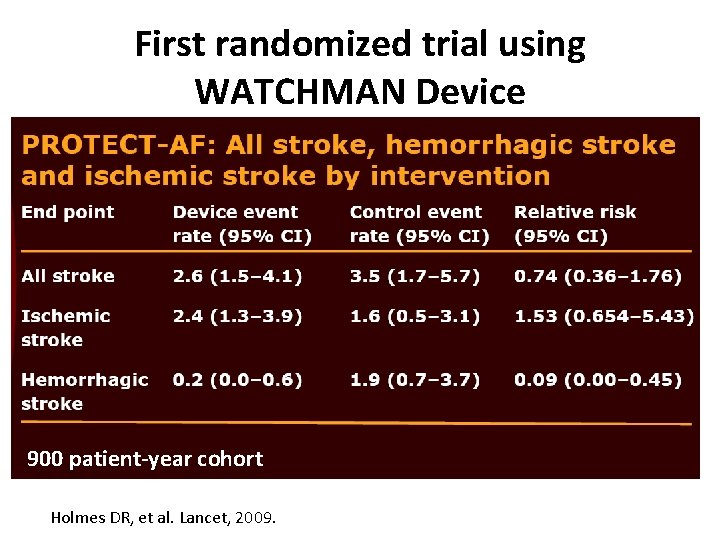
First randomized trial using WATCHMAN Device 900 patient-year cohort Holmes DR, et al. Lancet, 2009.
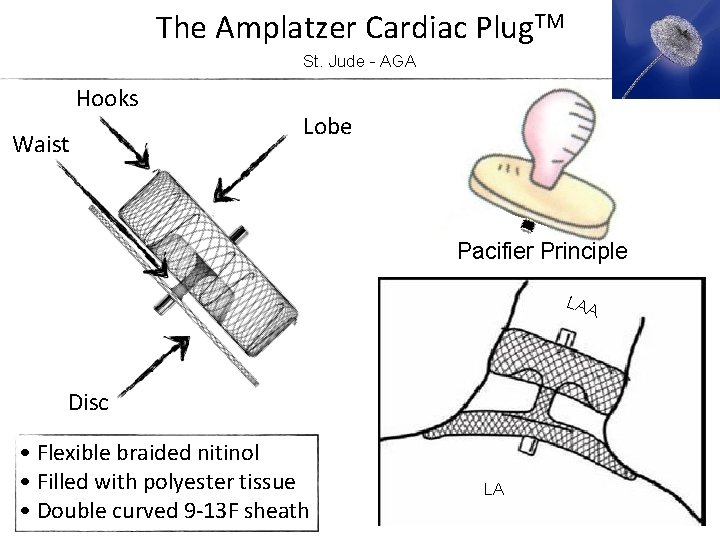
The Amplatzer Cardiac Plug. TM St. Jude - AGA Hooks Waist Lobe Pacifier Principle LAA Disc • Flexible braided nitinol • Filled with polyester tissue • Double curved 9 -13 F sheath LA
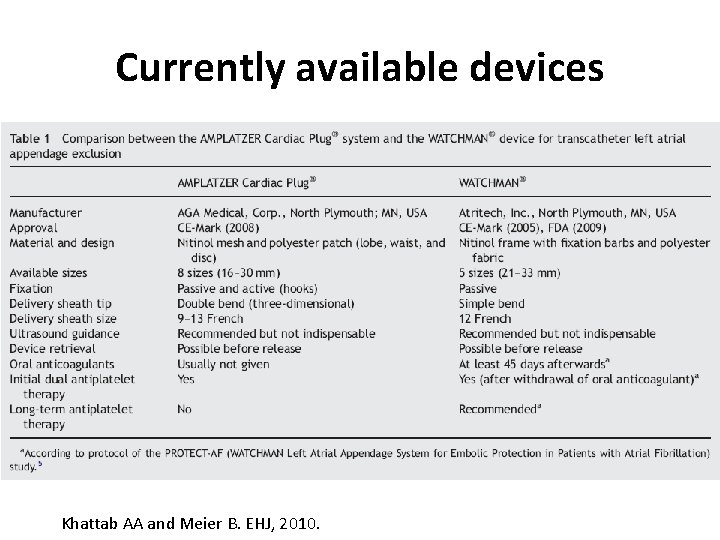
Currently available devices Khattab AA and Meier B. EHJ, 2010.
Khattab AA and Meier B. EHJ, 2010.
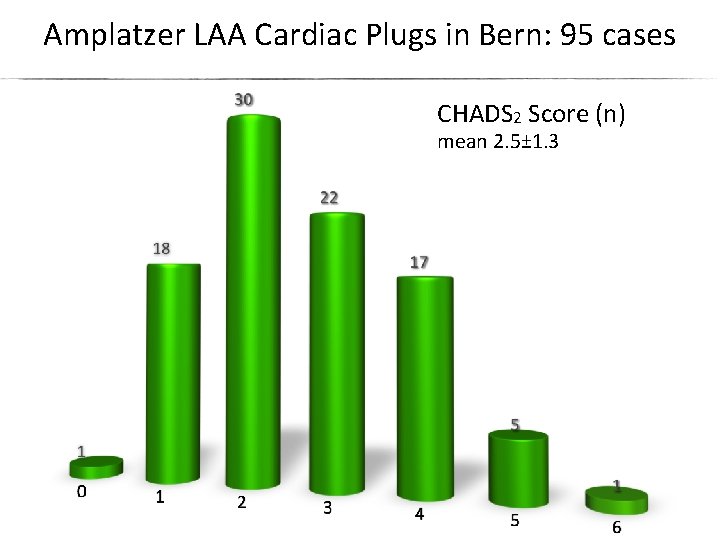
Amplatzer LAA Cardiac Plugs in Bern: 95 cases CHADS 2 Score (n) mean 2. 5± 1. 3
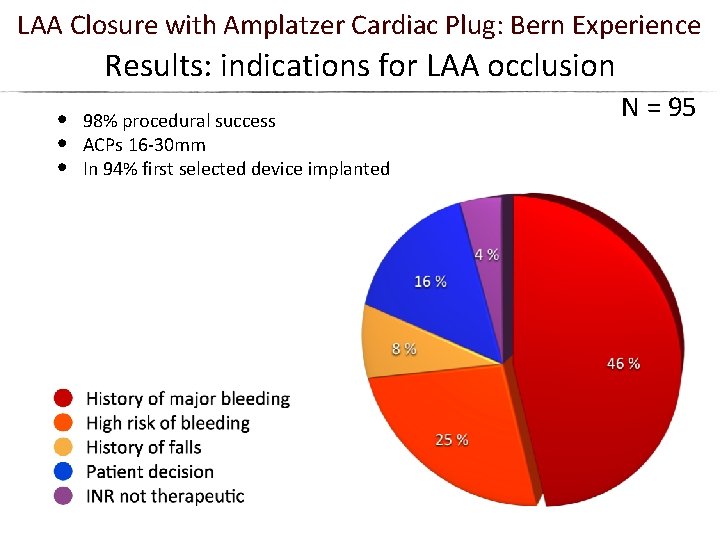
LAA Closure with Amplatzer Cardiac Plug: Bern Experience Results: indications for LAA occlusion • • • 98% procedural success ACPs 16 -30 mm In 94% first selected device implanted N = 95
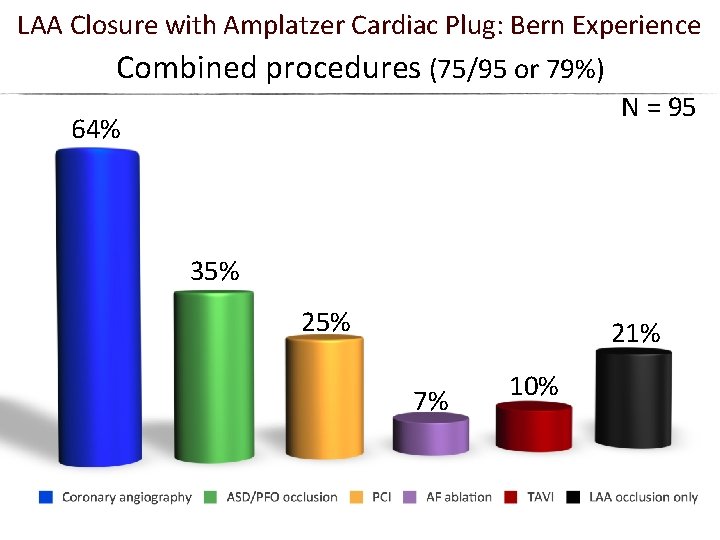
LAA Closure with Amplatzer Cardiac Plug: Bern Experience Combined procedures (75/95 or 79%) N = 95 64% 35% 21% 7% 10%
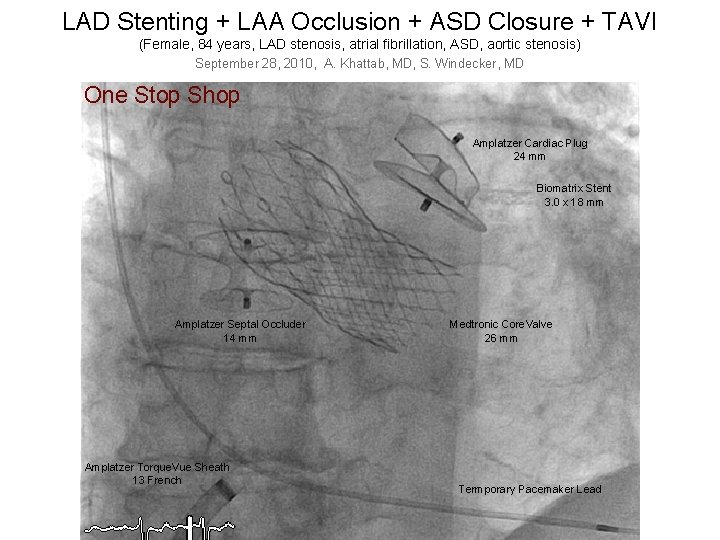
LAD Stenting + LAA Occlusion + ASD Closure + TAVI (Female, 84 years, LAD stenosis, atrial fibrillation, ASD, aortic stenosis) September 28, 2010, A. Khattab, MD, S. Windecker, MD One Stop Shop Amplatzer Cardiac Plug 24 mm Biomatrix Stent 3. 0 x 18 mm Schneiter Elisabeth, 29. 11. 1926, 28. 09. 2010, Khattab, Windecker Amplatzer Septal Occluder 14 mm Amplatzer Torque. Vue Sheath 13 French Medtronic Core. Valve 26 mm Termporary Pacemaker Lead
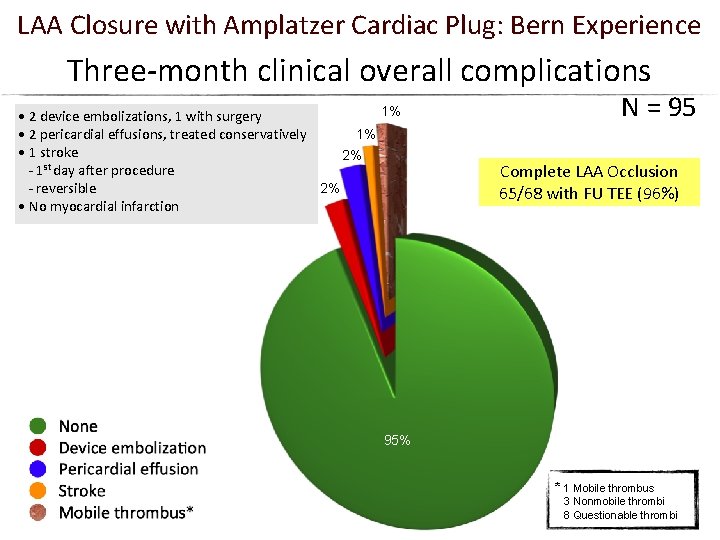
LAA Closure with Amplatzer Cardiac Plug: Bern Experience Three-month clinical overall complications 1% • 2 device embolizations, 1 with surgery 1% • 2 pericardial effusions, treated conservatively • 1 stroke 2% st - 1 day after procedure - reversible 2% • No myocardial infarction N = 95 Complete LAA Occlusion 65/68 with FU TEE (96%) 95% * 1 Mobile thrombus 3 Nonmobile thrombi 8 Questionable thrombi
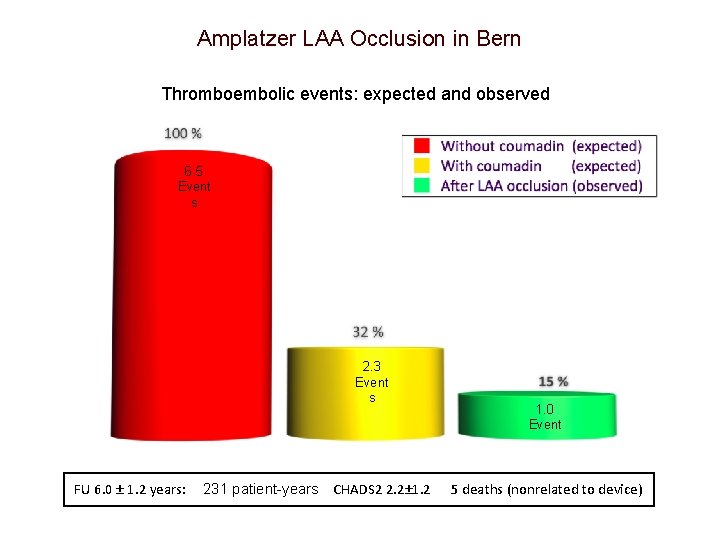
Amplatzer LAA Occlusion in Bern Thromboembolic events: expected and observed 6. 5 Event s 1. 0 Event 2. 3 Event s FU 6. 0 ± 1. 2 years: 231 patient-years CHADS 2 2. 2± 1. 2 1. 0 Event 5 deaths (nonrelated to device)

AMPLATZER® Cardiac Plug • CE Mark 2008 – Implanted since December 2008 • Several physician initiated studies – Pre-registry data – Park et al. (2008 -2009) – Italian Registry – Santoro et al. (2008 -2010) – Dual Center – Park, Meier (2010 -2011) • EU Post-Marketing Registry – First patient enrolled August 2009 – Enrollment completed September 2011 • US FDA Randomized Trial – Completed enrollment in feasibility phase (45 patients) – Up to 2000 patients to be enrolled in pivotal phase – expected start: Q 1 2012
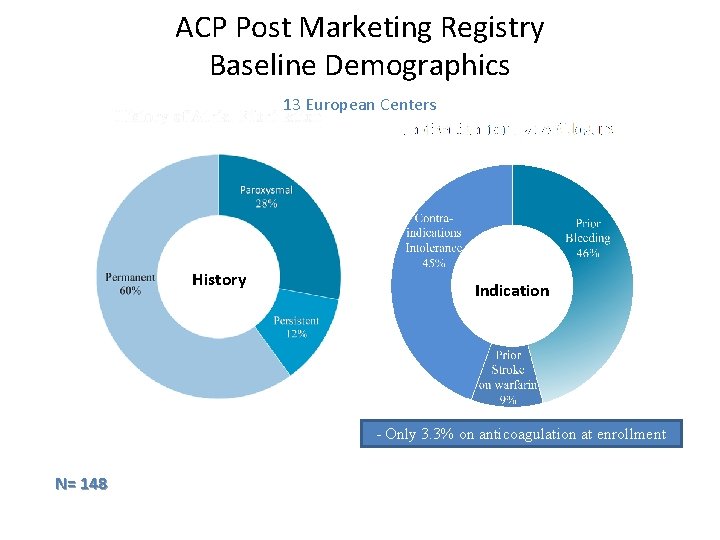
ACP Post Marketing Registry Baseline Demographics 13 European Centers History Indication - Only 3. 3% on anticoagulation at enrollment N= 148
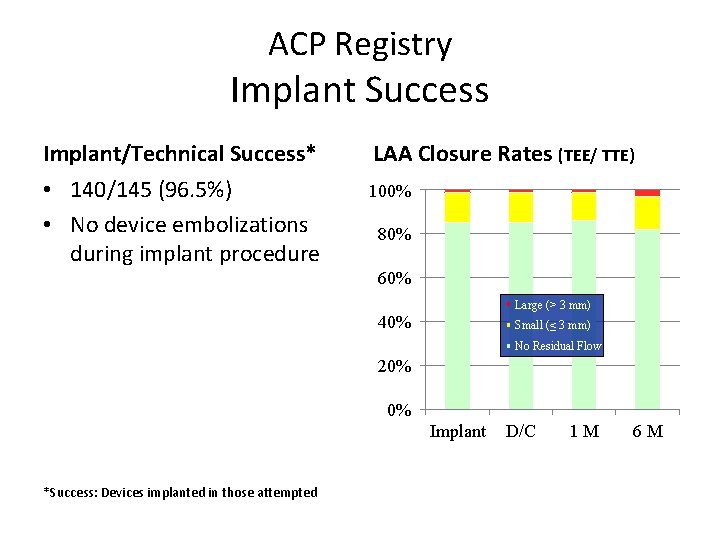
ACP Registry Implant Success Implant/Technical Success* LAA Closure Rates (TEE/ TTE) • 140/145 (96. 5%) • No device embolizations during implant procedure 100% 80% 60% Large (> 3 mm) 40% Small (≤ 3 mm) No Residual Flow 20% 0% N = 1 4 0 Implant *Success: Devices implanted in those attempted N = 1 4 0 D/C N = 1 2 9 1 M N = 8 7 6 M
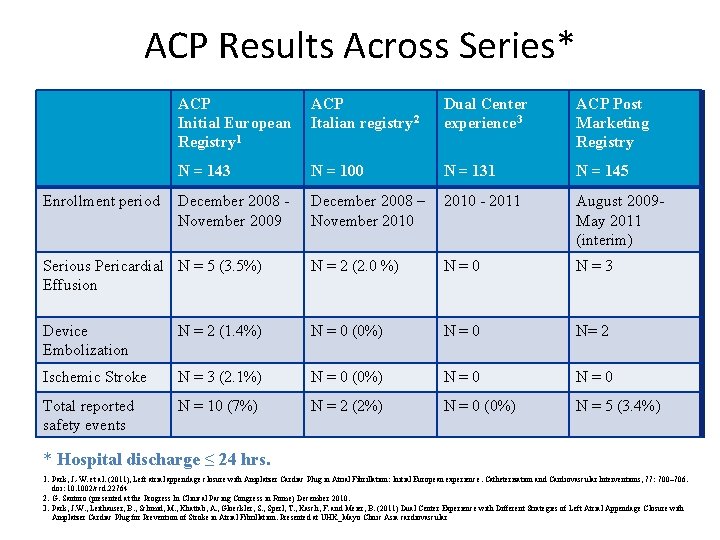
ACP Results Across Series* ACP Initial European Registry 1 ACP Italian registry 2 Dual Center experience 3 ACP Post Marketing Registry N = 143 N = 100 N = 131 N = 145 December 2008 November 2009 December 2008 – November 2010 - 2011 August 2009 May 2011 (interim) Serious Pericardial N = 5 (3. 5%) Effusion N = 2 (2. 0 %) N=0 N=3 Device Embolization N = 2 (1. 4%) N = 0 (0%) N=0 N= 2 Ischemic Stroke N = 3 (2. 1%) N = 0 (0%) N=0 Total reported safety events N = 10 (7%) N = 2 (2%) N = 0 (0%) N = 5 (3. 4%) Enrollment period * Hospital discharge ≤ 24 hrs. 1. Park, J. -W. et al. (2011), Left atrial appendage closure with Amplatzer Cardiac Plug in Atrial Fibrillation: Initial European experience. Catheterization and Cardiovascular Interventions, 77: 700– 706. doi: 10. 1002/ccd. 22764 2. G. Santoro (presented at the Progress In Clinical Pacing Congress in Rome) December 2010. 3. Park, J. W. , Leithauser, B. , Schmid, M. , Khattab, A. , Gloeckler, S. , Sperl, T. , Kasch, F. and Meier, B. (2011) Dual Center Experience with Different Strategies of Left Atrial Appendage Closure with Amplatzer Cardiac Plug for Prevention of Stroke in Atrial Fibrillation. Presented at UHK_Mayo Clinic Asia cardiovascular summit. 26 -7 March (Hong Kong).
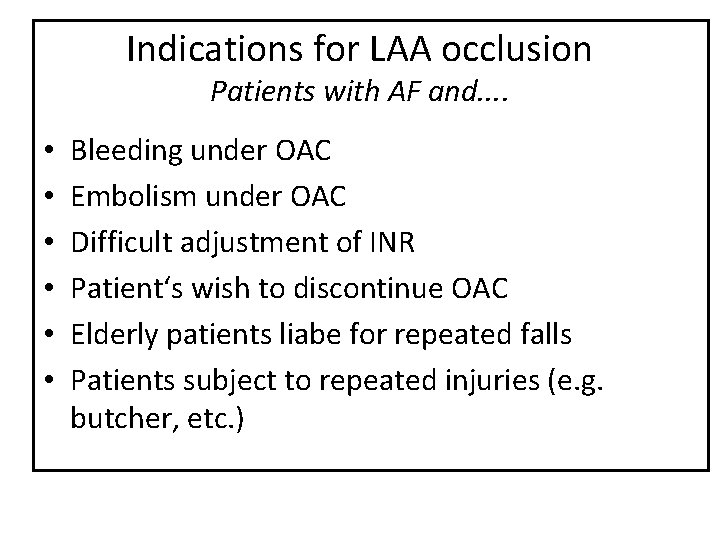
Indications for LAA occlusion Patients with AF and. . • • • Bleeding under OAC Embolism under OAC Difficult adjustment of INR Patient‘s wish to discontinue OAC Elderly patients liabe for repeated falls Patients subject to repeated injuries (e. g. butcher, etc. )
- Slides: 19