Transcatheter Aortic Valve Replacement Using the Lotus Valve



- Slides: 15

Transcatheter Aortic Valve Replacement Using the Lotus Valve with Depth Guard First Report from the RESPOND Extension Study Nicolas M Van Mieghem, MD, Ph. D Thoraxcenter, Erasmus MC, Rotterdam, the Netherlands Daniel J Blackman, MD; David Hildick-Smith, MD; Adam Witkowski, MD; Saib S Khogali, MD; Marek Grygier, MD; Dominic J Allocco, MD; Keith D Dawkins, MD

Disclosure I, Nicolas Van Mieghem, MD, Ph. D, has received research grant support from ü Abbott Vascular ü Boston Scientific ü Claret Medical ü Medtronic ü Pulse. Cath Inc.

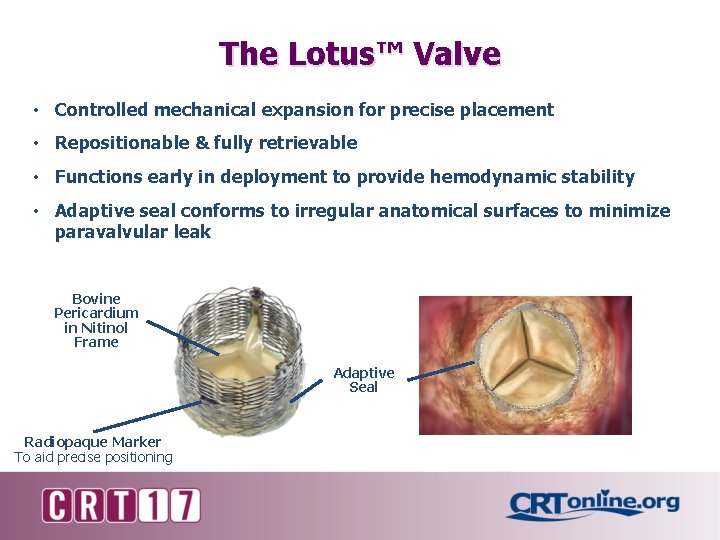
The Lotus™ Valve • Controlled mechanical expansion for precise placement • Repositionable & fully retrievable • Functions early in deployment to provide hemodynamic stability • Adaptive seal conforms to irregular anatomical surfaces to minimize paravalvular leak Bovine Pericardium in Nitinol Frame Adaptive Seal Radiopaque Marker To aid precise positioning

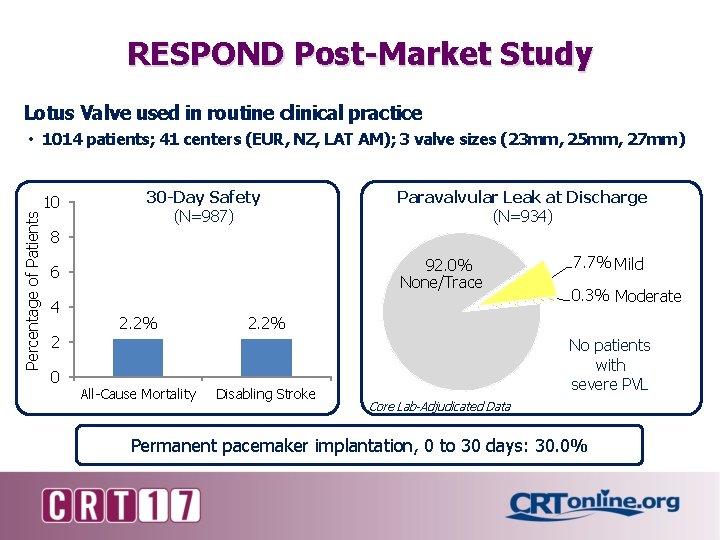
RESPOND Post-Market Study Lotus Valve used in routine clinical practice Percentage of Patients • 1014 patients; 41 centers (EUR, NZ, LAT AM); 3 valve sizes (23 mm, 25 mm, 27 mm) 10 30 -Day Safety (N=987) Paravalvular Leak at Discharge (N=934) 8 92. 0% None/Trace 6 4 2 0 2. 2% All-Cause Mortality 7. 7% Mild 0. 3% Moderate 2. 2% Disabling Stroke No patients with severe PVL Core Lab-Adjudicated Data Permanent pacemaker implantation, 0 to 30 days: 30. 0%

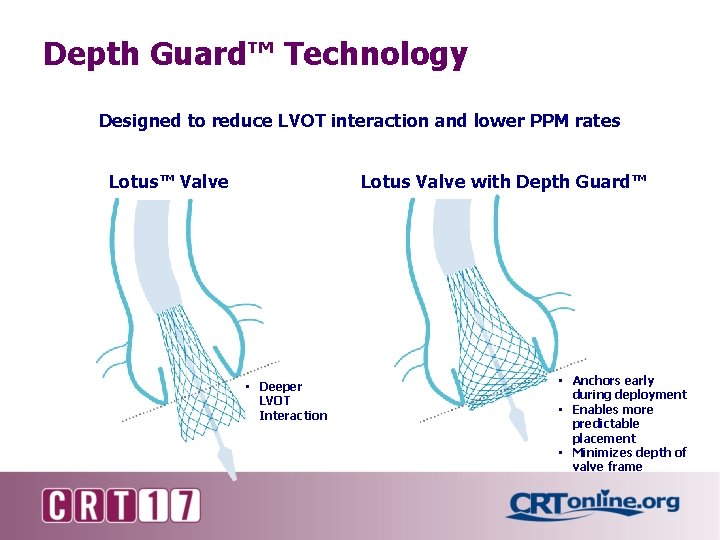
Depth Guard™ Technology Designed to reduce LVOT interaction and lower PPM rates Lotus™ Valve Lotus Valve with Depth Guard™ • Deeper LVOT Interaction • Anchors early during deployment • Enables more predictable placement • Minimizes depth of valve frame

LOTUS with Depth Guard™ Depth Guard optimizes valve deployment: Lotus Valve LOTUS Edge System + Depth Guard • Anchors early during deployment • Minimizes depth of valve frame, reducing interaction with the LVOT & conduction system • When used in combination with modifications to valve implant technique, may reduce the need for new pacemaker implantation

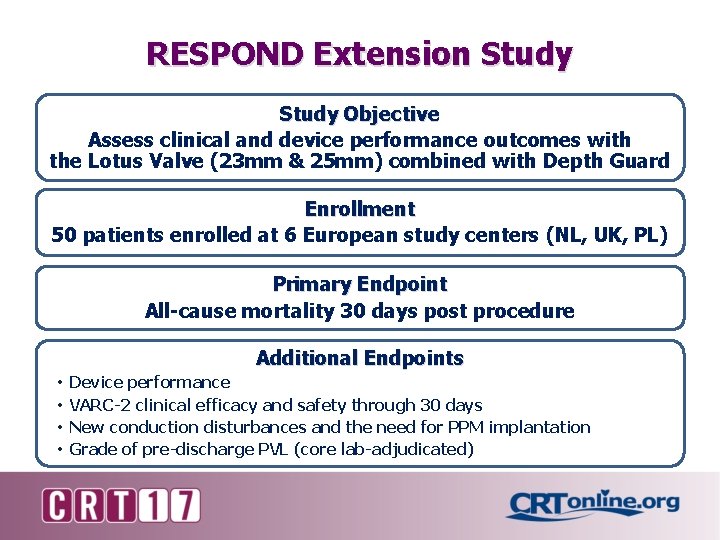
RESPOND Extension Study Objective Assess clinical and device performance outcomes with the Lotus Valve (23 mm & 25 mm) combined with Depth Guard Enrollment 50 patients enrolled at 6 European study centers (NL, UK, PL) Primary Endpoint All-cause mortality 30 days post procedure Additional Endpoints • • Device performance VARC-2 clinical efficacy and safety through 30 days New conduction disturbances and the need for PPM implantation Grade of pre-discharge PVL (core lab-adjudicated)

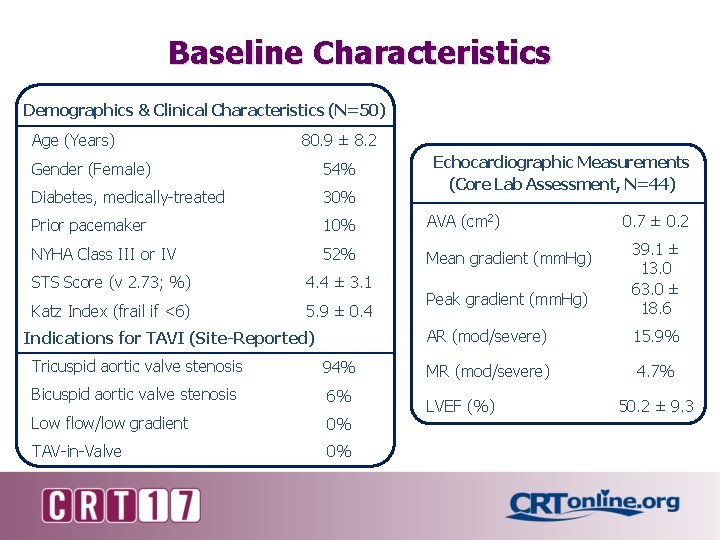
Baseline Characteristics Demographics & Clinical Characteristics (N=50) Age (Years) 80. 9 ± 8. 2 Echocardiographic Measurements (Core Lab Assessment, N=44) Gender (Female) 54% Diabetes, medically-treated 30% Prior pacemaker 10% AVA (cm 2) NYHA Class III or IV 52% Mean gradient (mm. Hg) STS Score (v 2. 73; %) 4. 4 ± 3. 1 Katz Index (frail if <6) 5. 9 ± 0. 4 Indications for TAVI (Site-Reported) Tricuspid aortic valve stenosis 94% Bicuspid aortic valve stenosis 6% Low flow/low gradient 0% TAV-in-Valve 0% Peak gradient (mm. Hg) 0. 7 ± 0. 2 39. 1 ± 13. 0 63. 0 ± 18. 6 AR (mod/severe) 15. 9% MR (mod/severe) 4. 7% LVEF (%) 50. 2 ± 9. 3

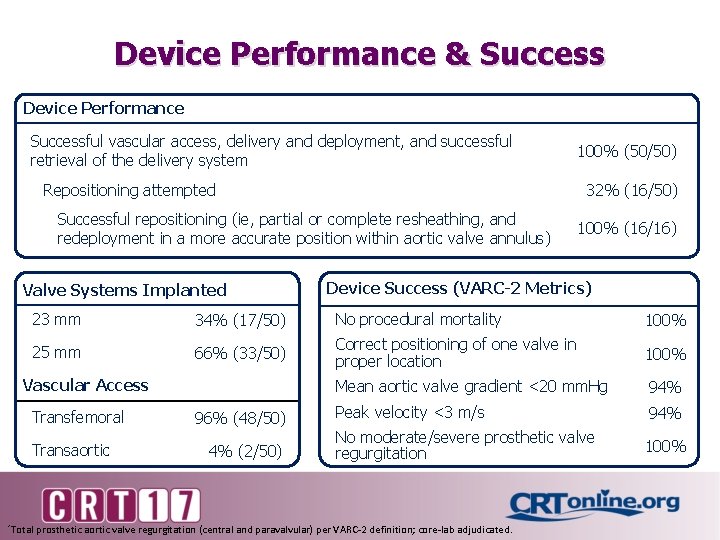
Device Performance & Success Device Performance Successful vascular access, delivery and deployment, and successful retrieval of the delivery system 100% (50/50) Repositioning attempted 32% (16/50) Successful repositioning (ie, partial or complete resheathing, and redeployment in a more accurate position within aortic valve annulus) Valve Systems Implanted 100% (16/16) Device Success (VARC-2 Metrics) 23 mm 34% (17/50) No procedural mortality 100% 25 mm 66% (33/50) Correct positioning of one valve in proper location 100% Mean aortic valve gradient <20 mm. Hg 94% Peak velocity <3 m/s 94% No moderate/severe prosthetic valve regurgitation 100% Vascular Access Transfemoral Transaortic 96% (48/50) 4% (2/50) *Total prosthetic aortic valve regurgitation (central and paravalvular) per VARC-2 definition; core-lab adjudicated.

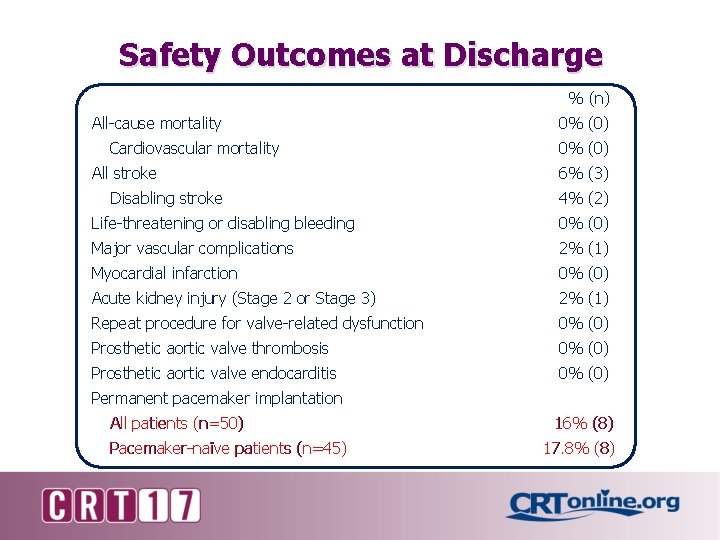
Safety Outcomes at Discharge % (n) All-cause mortality Cardiovascular mortality All stroke Disabling stroke 0% (0) 6% (3) 4% (2) Life-threatening or disabling bleeding 0% (0) Major vascular complications 2% (1) Myocardial infarction 0% (0) Acute kidney injury (Stage 2 or Stage 3) 2% (1) Repeat procedure for valve-related dysfunction 0% (0) Prosthetic aortic valve thrombosis 0% (0) Prosthetic aortic valve endocarditis 0% (0) Permanent pacemaker implantation All patients (n=50) Pacemaker-naïve patients (n=45) 16% (8) 17. 8% (8)

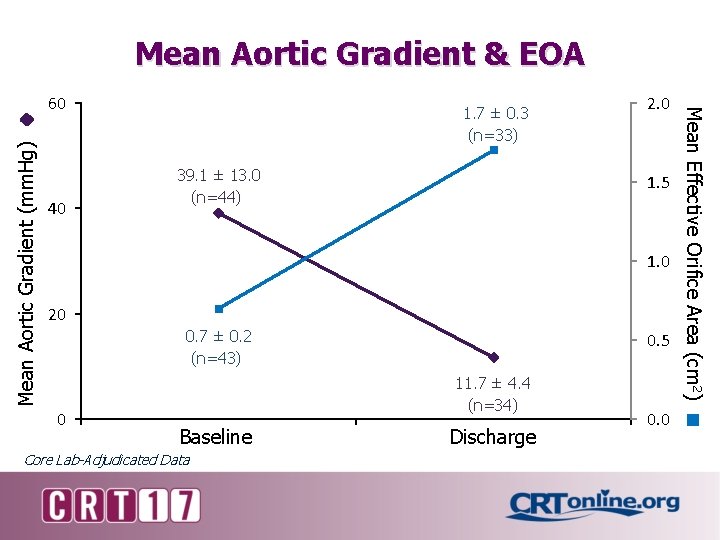
Mean Aortic Gradient & EOA Mean Aortic Gradient (mm. Hg) 40 1. 7 ± 0. 3 (n=33) 39. 1 ± 13. 0 (n=44) 2. 0 1. 5 1. 0 20 0. 7 ± 0. 2 (n=43) 0 0. 5 11. 7 ± 4. 4 (n=34) Baseline Core Lab-Adjudicated Data Discharge 0. 0 Mean Effective Orifice Area (cm 2) 60

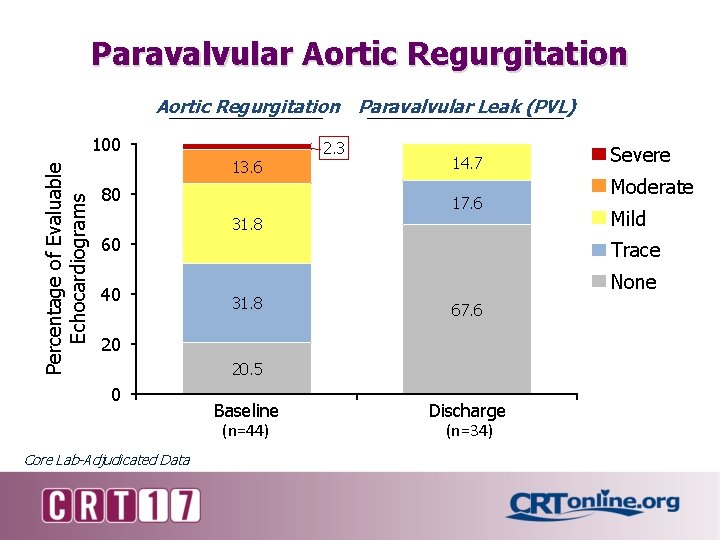
Paravalvular Aortic Regurgitation Paravalvular Leak (PVL) Percentage of Evaluable Echocardiograms 100 13. 6 80 2. 3 14. 7 17. 6 31. 8 60 40 Mild 31. 8 None 67. 6 20. 5 Core Lab-Adjudicated Data Moderate Trace 20 0 Severe Baseline (n=44) Discharge (n=34)

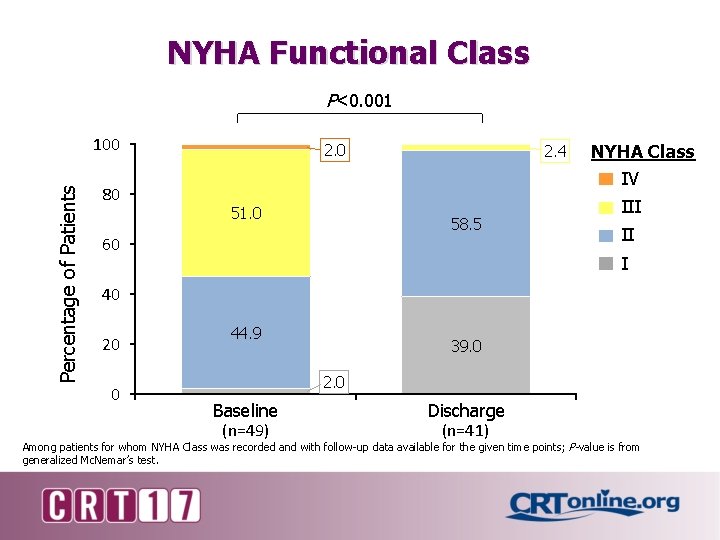
NYHA Functional Class P<0. 001 Percentage of Patients 100 80 2. 0 51. 0 2. 4 58. 5 60 NYHA Class IV III II I 40 20 0 44. 9 39. 0 2. 0 Baseline (n=49) Discharge (n=41) Among patients for whom NYHA Class was recorded and with follow-up data available for the given time points; P-value is from generalized Mc. Nemar’s test.

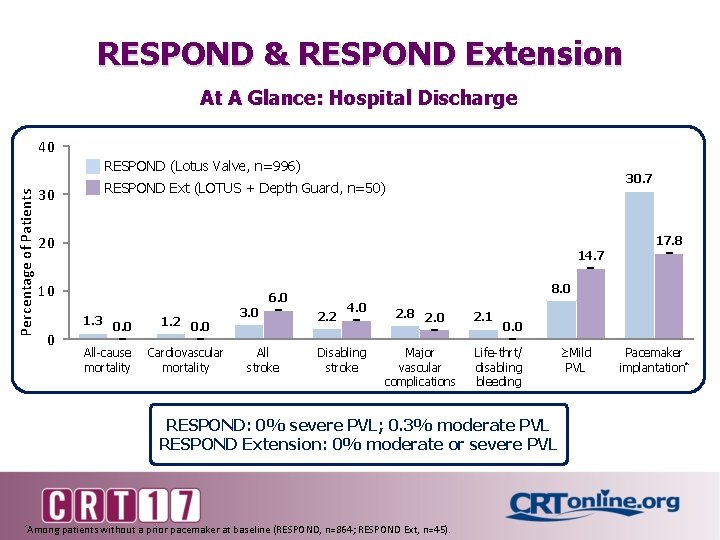
RESPOND & RESPOND Extension At A Glance: Hospital Discharge 40 Percentage of Patients RESPOND (Lotus Valve, n=996) 30. 7 RESPOND Ext (LOTUS + Depth Guard, n=50) 30 20 17. 8 14. 7 10 1. 3 0 8. 0 6. 0 0. 0 All-cause mortality 1. 2 0. 0 Cardiovascular mortality 3. 0 All stroke 2. 2 4. 0 Disabling stroke 2. 8 2. 0 Major vascular complications 2. 1 0. 0 Life-thrt/ disabling bleeding RESPOND: 0% severe PVL; 0. 3% moderate PVL RESPOND Extension: 0% moderate or severe PVL *Among patients without a prior pacemaker at baseline (RESPOND, n=864; RESPOND Ext, n=45). ≥Mild PVL Pacemaker implantation*

Conclusions The RESPOND Extension study establishes the early safety and efficacy of TAVI with the Lotus Valve with Depth Guard • 0% mortality at discharge/7 days • Low paravalvular regurgitation (core lab-adjudicated) • 85. 3% of patients with no/trivial PVL • No patients with moderate or severe PVL • Permanent pacemaker implantation (0 -7 d/discharge) • 16% among all patients; 17. 7% among pacemaker-naïve patients Data on 30 -day outcomes from RESPOND Extension are expected to be reported at Euro. PCR 2017