Trans Effect in Ligand Substitution Reactions In Square
Trans Effect in Ligand Substitution Reactions In Square Planar Complexes Dr. Rajesh Kumar Malik Dept. of Chemistry M. D. U. Rohtak
Substitution Reactions of Sq. Pl. Complexes • Ligand substitution reactions in square planar complexes • dsp 2 - hybridization, in square planar complexes, the central metal is dsp 2 hybridized with co. no. 4 • Associative or inter change associative type mechanism • Berry pseudo rotation • Square planar Pt(II) complexes as anticancer drugs • Solvent molecules association as ligand • Retention of configuration. • Usually d 8 -metal ions form square planar complexes. i. e. 4 ligands are arranged around the metal ion at angle of 90 o in one plane. Co+, e. g. Rh+, Ir+, Ni 2+, Pd 2+, Pt 2+, Ag 3+, Cu 3+ and Au 3+ etc.
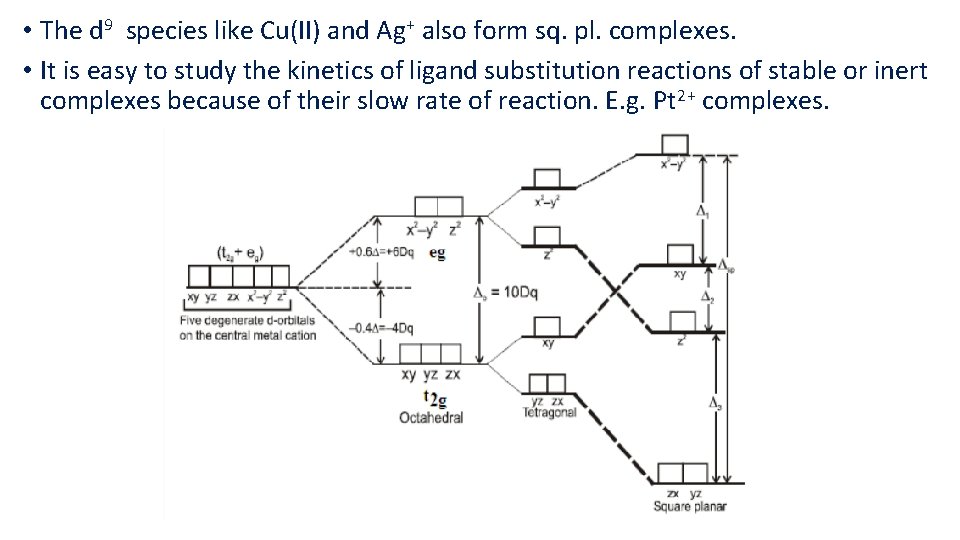
• The d 9 species like Cu(II) and Ag+ also form sq. pl. complexes. • It is easy to study the kinetics of ligand substitution reactions of stable or inert complexes because of their slow rate of reaction. E. g. Pt 2+ complexes.

TRANS - EFFECT

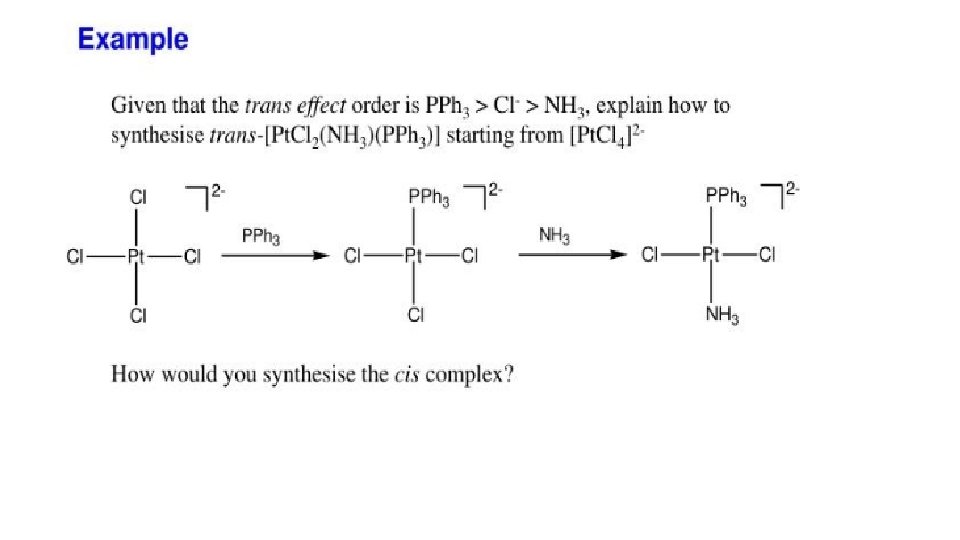
TRANS EFFECT AND ITS APPLICATIONS : “The ability of a ligand in square planar complex to direct the replacement of ligand present at its trans position is known as trans effect. ” Ø The stronger trans directing ligand, increases lability of the ligand present at its trans position in square planar complex for a substitution reaction. Ø This is very much useful in the synthesis of square planar complexes. Ø The ligands are arranged in the increasing order of trans effect as follows:


Π- Acid ligands show stronger trans effect due to pπ-dπ back bonding ability of metal with vacant ABMO of ligand. E. g. CO > CN- C 2 H 4 > PR 3
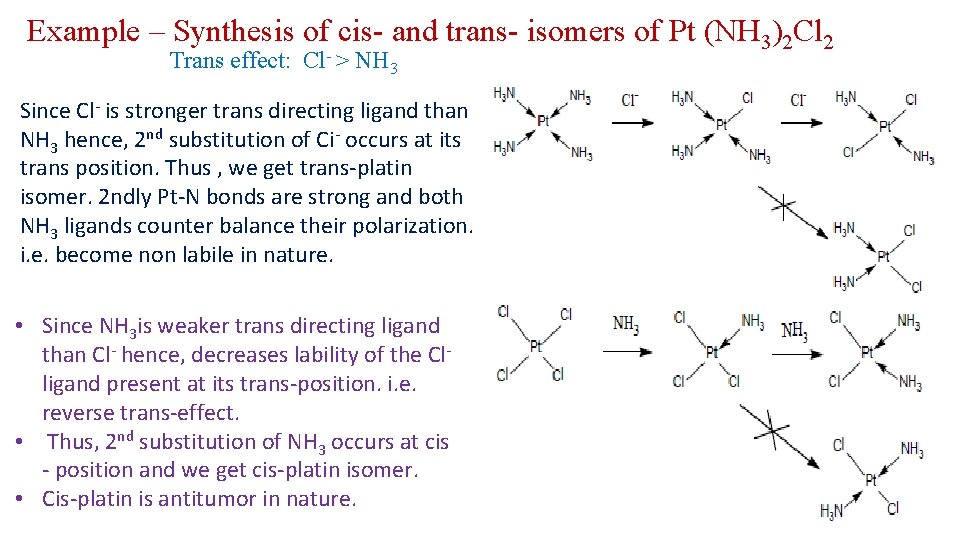
Example – Synthesis of cis- and trans- isomers of Pt (NH 3)2 Cl 2 Trans effect: Cl- > NH 3 Since Cl- is stronger trans directing ligand than NH 3 hence, 2 nd substitution of Ci- occurs at its trans position. Thus , we get trans-platin isomer. 2 ndly Pt-N bonds are strong and both NH 3 ligands counter balance their polarization. i. e. become non labile in nature. • Since NH 3 is weaker trans directing ligand than Cl- hence, decreases lability of the Clligand present at its trans-position. i. e. reverse trans-effect. • Thus, 2 nd substitution of NH 3 occurs at cis - position and we get cis-platin isomer. • Cis-platin is antitumor in nature.

APPLICATIONS OF TRANS EFFECT: •
![[2]Synthesis of Cis- and Trans- [Pt(Cl)2 NO 2 NH 3] starting from tetrachloridoplatinate(ll) ion. [2]Synthesis of Cis- and Trans- [Pt(Cl)2 NO 2 NH 3] starting from tetrachloridoplatinate(ll) ion.](http://slidetodoc.com/presentation_image_h2/5b0aef9fc7689f30e228dc62e65339e2/image-11.jpg)
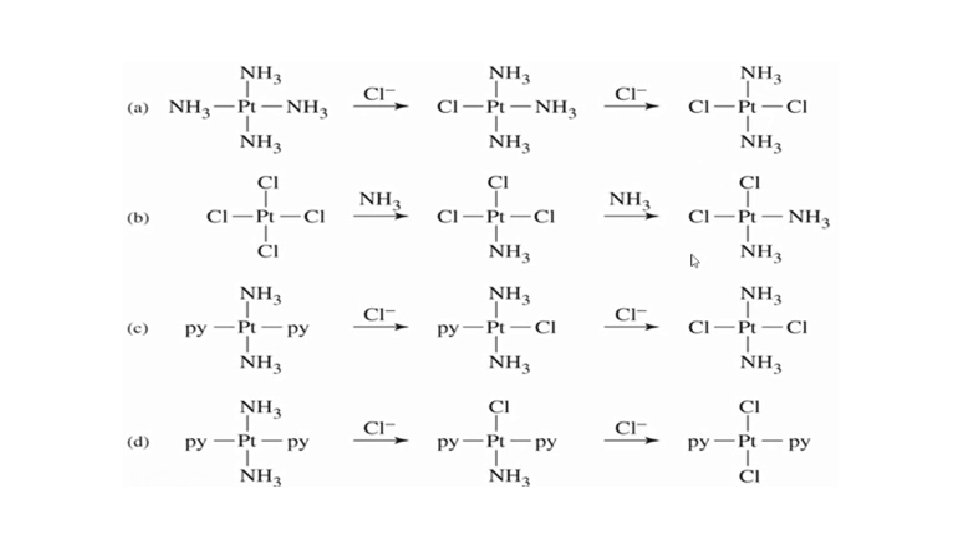
[2]Synthesis of Cis- and Trans- [Pt(Cl)2 NO 2 NH 3] starting from tetrachloridoplatinate(ll) ion. The trans directing nature of ligands is NO 2 -> Cl- > NH 3. Depends on order in which the reagents are added as to which geometrical isomer is formed so, has used for devising synthesis of Pt(II) complexes. The phenomenon of trans effect can utilize for the synthesis of isomeric compounds. The Trans effect is increasing in the order of NO 2 - > Cl- > NH 3. Hence the synthesis of cis- and trans- isomer can be followed as:

![[4]Synthesis of cis- and trans-[Pt(C 2 H 4)(NH 3)Cl 2] complex starting from chlorocomplex [4]Synthesis of cis- and trans-[Pt(C 2 H 4)(NH 3)Cl 2] complex starting from chlorocomplex](http://slidetodoc.com/presentation_image_h2/5b0aef9fc7689f30e228dc62e65339e2/image-13.jpg)
[4]Synthesis of cis- and trans-[Pt(C 2 H 4)(NH 3)Cl 2] complex starting from chlorocomplex of Pt(II): trans- effect order is C 2 H 4 > Cl- > NH 3 Depends on order in which the reagents are added as to which geometrical isomer is formed so, has used for devising synthesis of Pt(II) complexes. i. e. Following different substitution order of different ligands we get different isomers of Pt(II). • If NH 3 is substituted 1 stly then it will show reverse trans effect for 2 nd substitution to form cis- isomer.
![[5]To distinguish Cis- and Trans- isomers of [Pt(Cl)2(NH 3)2]. These can be distinguished by [5]To distinguish Cis- and Trans- isomers of [Pt(Cl)2(NH 3)2]. These can be distinguished by](http://slidetodoc.com/presentation_image_h2/5b0aef9fc7689f30e228dc62e65339e2/image-15.jpg)
[5]To distinguish Cis- and Trans- isomers of [Pt(Cl)2(NH 3)2]. These can be distinguished by Kurnakove’s test using thiourea ligand: It is used to distinguish cis- and trans-isomers of a complex compound. The cis- and trans isomers react with thiourea(NH 2 -CS-NH 2) in different molar ratio to give different products. • Cis-Platin reacts with 4 mol thiourea, gives a yellow water soluble product. [Pt(Tu)4]Cl 2 • Trans-Platin with 2 mol thiourea, gives a white partially water soluble product. [Pt(Tu)4]Cl 2 Pt-N is stronger bond and two NH 3 ligands at trans position do not show labialisation, hence trans-Platin consumes 2 -mol thiourea.
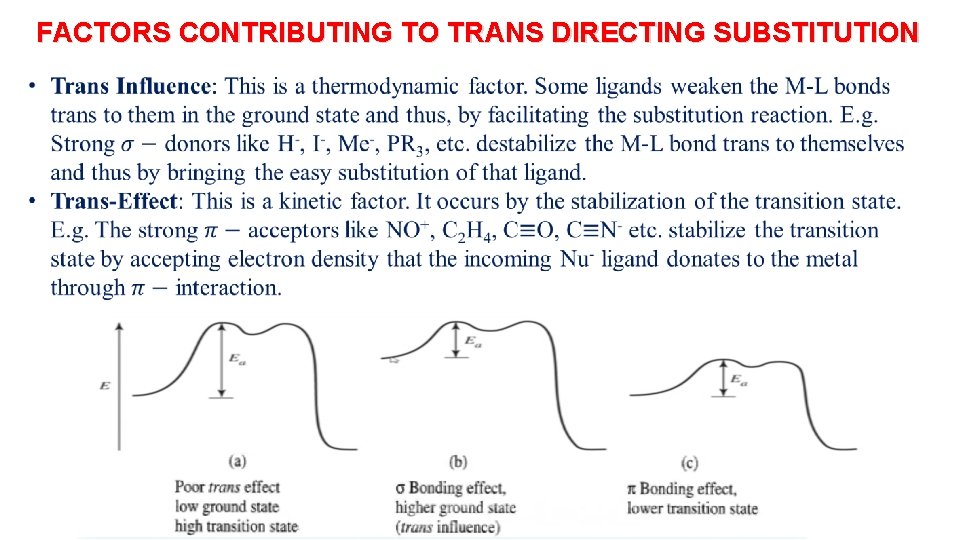
FACTORS CONTRIBUTING TO TRANS DIRECTING SUBSTITUTION
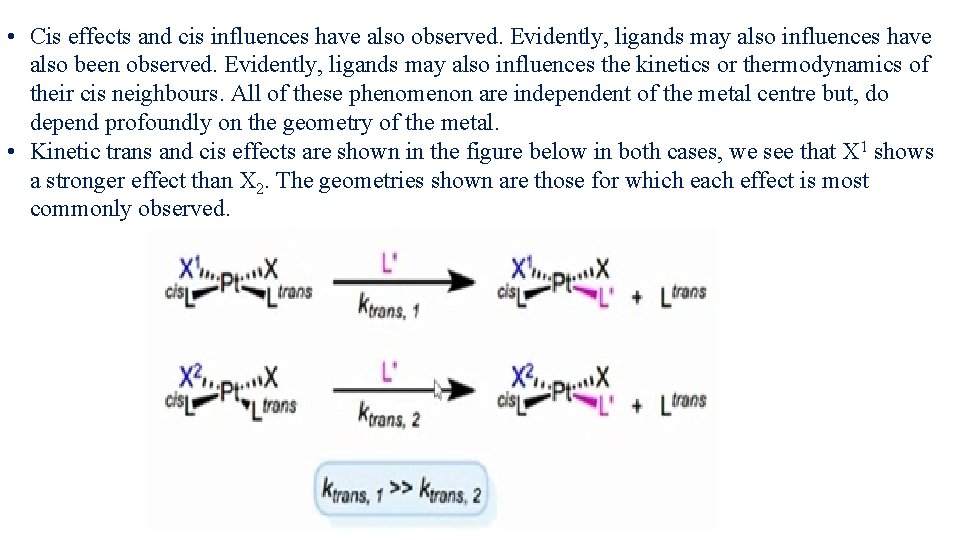
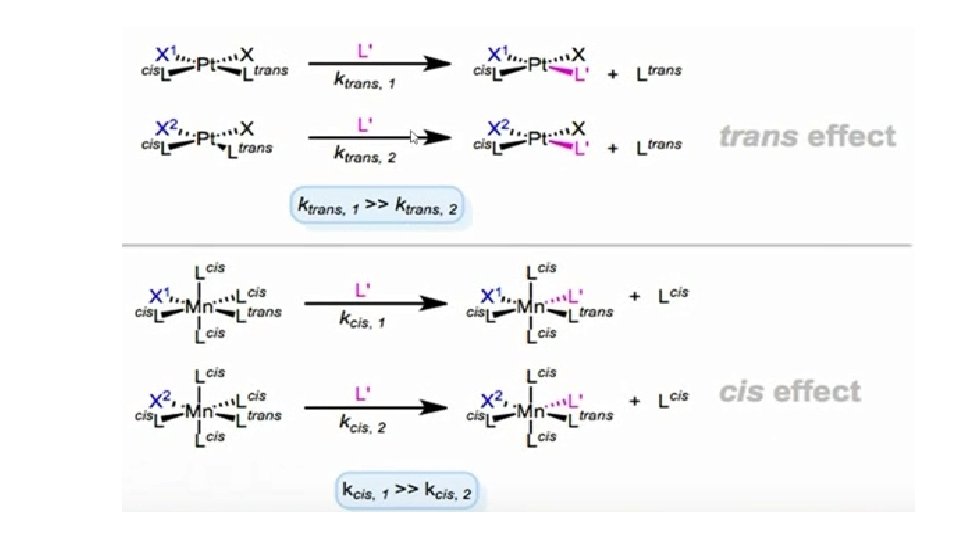
• Cis effects and cis influences have also observed. Evidently, ligands may also influences have also been observed. Evidently, ligands may also influences the kinetics or thermodynamics of their cis neighbours. All of these phenomenon are independent of the metal centre but, do depend profoundly on the geometry of the metal. • Kinetic trans and cis effects are shown in the figure below in both cases, we see that X 1 shows a stronger effect than X 2. The geometries shown are those for which each effect is most commonly observed.
Theories of Trans Effect • Electrostatic Polarization Theory: By Grinberg • Pi-Bonding Theory: By Chatt Orgel
![[1] Polarization Theory ( ground state effect ): By Grinberg • In homoleptic square [1] Polarization Theory ( ground state effect ): By Grinberg • In homoleptic square](http://slidetodoc.com/presentation_image_h2/5b0aef9fc7689f30e228dc62e65339e2/image-20.jpg)
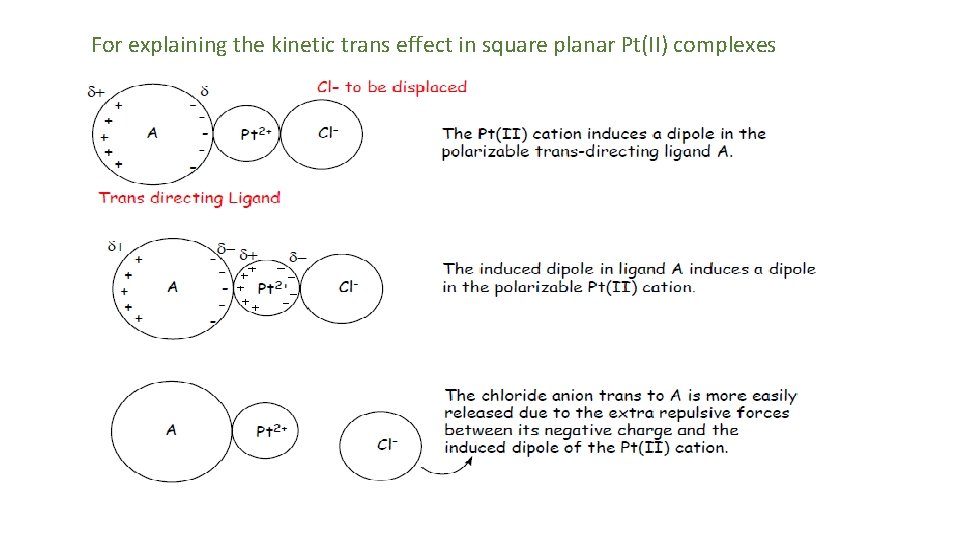
[1] Polarization Theory ( ground state effect ): By Grinberg • In homoleptic square planar complexes like [Pt(Cl)4]2 - all the M-L bonds are equally polarizes due to symmetrical nature hence net dipole becomes zero and trans effect is not observed. • The ligands of larger size get polarise toward metal centre hence, polarization of M —L bond weakens the M—X bond at its trans position in the complexes like [Pt. L 3 X]. Thus, M-X bond length increases and facilitates the departure of ligand-X or makes labile nature of ligand X- at trans position.

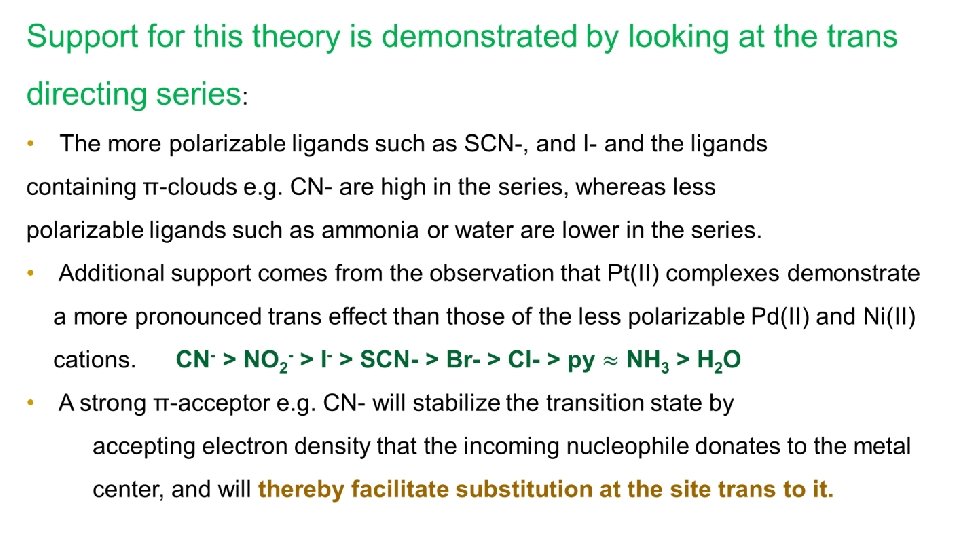
Support for this theory is demonstrated by looking at the trans directing series. The more polarizable ligands such as SCN-, and I- and the ligand containing π-clouds e. g. CN- are high in the series, whereas less polarizable ligands such as ammonia or water are lower in the series. Additional support comes from the observation that Pt(II) complexes demonstrate a more pronounced trans effect than those of the less polarizable Pd(II) and Ni(II) cations.
For explaining the kinetic trans effect in square planar Pt(II) complexes
Other contributing factors to the trans-effect: • In the trigonal plane of the 5 -coordinate transition state or intermediate, a πbonding interaction can occur between a metal d-orbital (e. g. dxy) and suitable orbitals (p atomic orbitals, or molecular orbitals of p-symmetry) of ligand L 2 (the ligand trans to the leaving group) and Y (the entering group). • These 3 ligands and the metal center can communicate electronically through π bonding only if they all lie in the same plane in the transition state or intermediate. • This implies the 5 -coordinate species must be trigonal pyramidal.

![[2] Pi-Bonding Theory: By Chatt-Orgel In chemistry, pi bonds (π bonds) are covalent bonds [2] Pi-Bonding Theory: By Chatt-Orgel In chemistry, pi bonds (π bonds) are covalent bonds](http://slidetodoc.com/presentation_image_h2/5b0aef9fc7689f30e228dc62e65339e2/image-27.jpg)
[2] Pi-Bonding Theory: By Chatt-Orgel In chemistry, pi bonds (π bonds) are covalent bonds which forms by lateral or side-wise overlap of the orbital of metal centre(dx 2 -y 2, dzx & dyz) dyz and the ligand. The strong π-acceptors (eg. CO, C 2 H 4, NO+ etc. ) remove electron density in the equatorial plane of 5 -coordinate tbp transition states thus decreasing electrostatic repulsion. E. g. [Pt. L 3 X] when one ligand L – is π-acceptor ligand. In [Pt. LX 3] type complexes let L 2 is π- acid ligand which forms dπ-pπ back bonding with metal centre , it results decrease in electron density at its trans position. Hence, weaken the Pt-X bond at trans-position of π- acid ligand-X becomes labile. The weaker Pt-X bond get lengthen and facilitates attack of new ligand to form penta coordinated TBPy transition state.
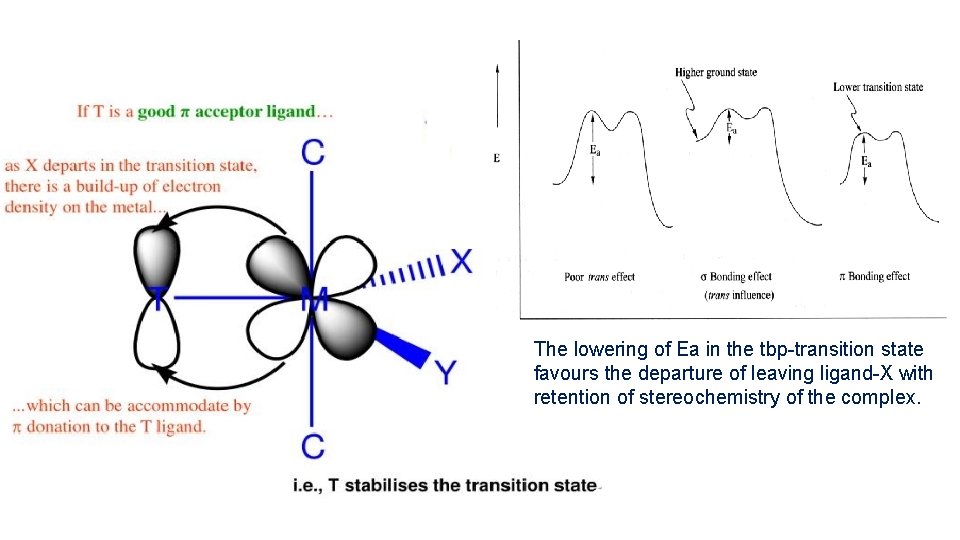
The lowering of Ea in the tbp-transition state favours the departure of leaving ligand-X with retention of stereochemistry of the complex.
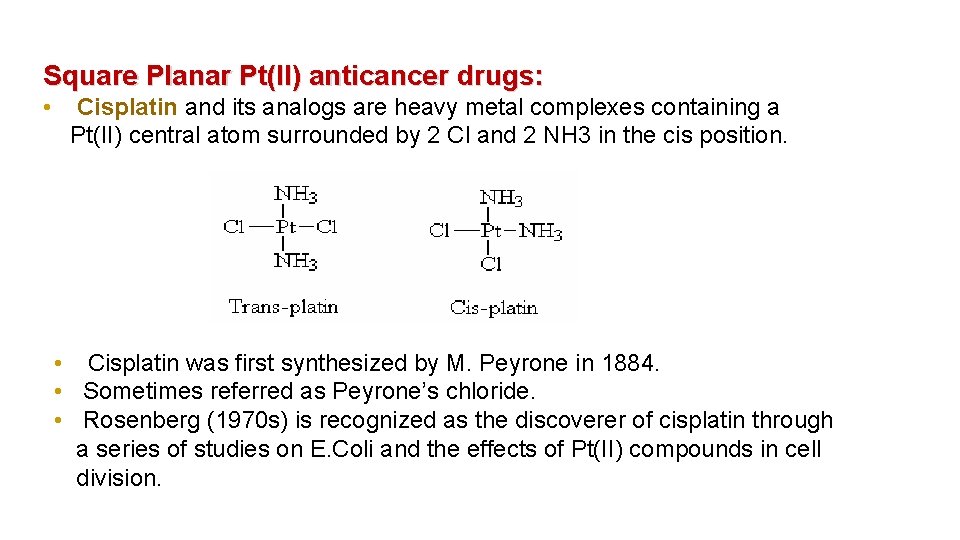
Square Planar Pt(II) anticancer drugs: • Cisplatin and its analogs are heavy metal complexes containing a Pt(II) central atom surrounded by 2 Cl and 2 NH 3 in the cis position. • Cisplatin was first synthesized by M. Peyrone in 1884. • Sometimes referred as Peyrone’s chloride. • Rosenberg (1970 s) is recognized as the discoverer of cisplatin through a series of studies on E. Coli and the effects of Pt(II) compounds in cell division.
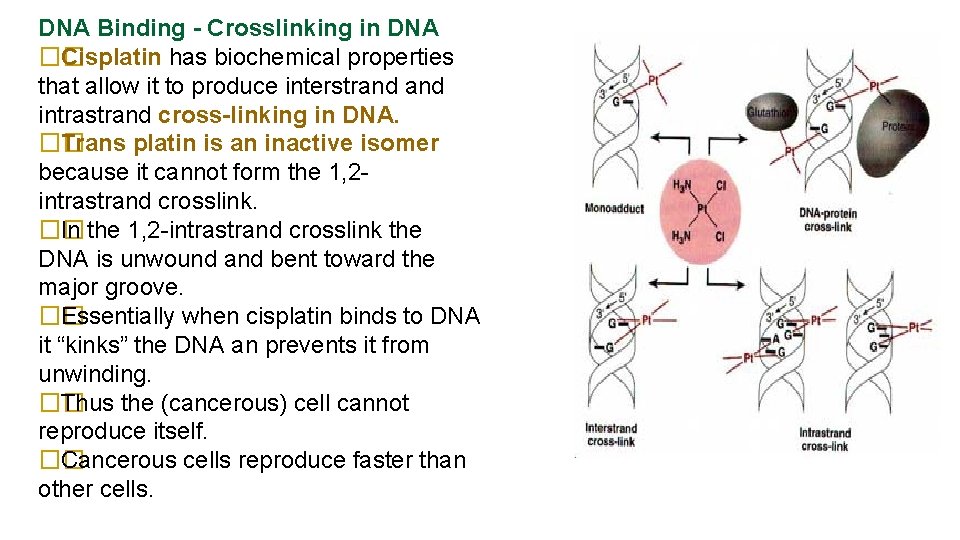
DNA Binding - Crosslinking in DNA �� Cisplatin has biochemical properties that allow it to produce interstrand intrastrand cross-linking in DNA. �� Trans platin is an inactive isomer because it cannot form the 1, 2 intrastrand crosslink. �� In the 1, 2 -intrastrand crosslink the DNA is unwound and bent toward the major groove. �� Essentially when cisplatin binds to DNA it “kinks” the DNA an prevents it from unwinding. �� Thus the (cancerous) cell cannot reproduce itself. �� Cancerous cells reproduce faster than other cells.

Thank You
- Slides: 31