TRANQUILIZING AGENT A sedative or tranquilizer is a










- Slides: 21

TRANQUILIZING AGENT • A sedative or tranquilizer is a substance that induces sedation by reducing irritability or excitement. Having a soothing, calming, or tranquilizing effect; reducing or relieving anxiety, stress, irritability, or excitement. • A traditional grouping of drugs said to have a soothing or calming effect on mood, thought, or behavior. Included here are the antianxiety agents (minor tranquilizers), antimanic agents, and the antipsychotic agents (major tranquilizers). These drugs act by different mechanisms and are used for different therapeutic purposes.

• Anti-Anxiety Agents: Agents that alleviate anxiety, tension, and anxiety disorders, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. Some are also effective as anticonvulsants, muscle relaxants, or anesthesia adjuvants. Adrenergic betaantagonists are commonly used in the symptomatic treatment of anxiety. • Antimanic Agents: Agents that are used to treat bipolar disorders or mania associated with other affective disorders. • Antipsychotic Agents: Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in schizophrenia, senile dementia, transient psychosis following surgery or myocardial infarction, etc. These drugs are often referred to as neuroleptics. Many of these drugs may also be effective against nausea, emesis, and pruritus.

Classification of Anxiolytic tranquilizers: • Benzodiazepines. This is the most important group, used as anxiolytic and hypnotic agents. • Buspirone. This 5 -HT 1 A receptor agonist is anxiolytic but not appreciably sedative. • β-Adrenoceptor antagonists (e. g. propranolol; ). These are used to treat some forms of anxiety, particularly where physical symptoms such as sweating, tremor and tachycardia are troublesome. • Zolpidem. This hypnotic acts similarly to benzodiazepines, although chemically distinct, but lacks appreciable anxiolytic activity. • Barbiturates. These are now largely obsolete, superseded by benzodiazepines. Their use is now confined to anaesthesia and the treatment of epilepsy. • Miscellaneous other drugs (e. g. chloral hydrate , meprobamate and methaqualone). They are no longer recommended, but therapeutic habits die hard and they are occasionally used.

Classification of Antipsychotic tranquilizers Antipsychotics may be classified under the following categories, namely : • (a) Reserpine and Related Alkaloids • (b) Alkylene Diols • (c) Diphenylmethane Compounds • (d) Phenothiazine Compounds • (e) Dibenzazepines • (f) Butyrophenones • (g) Azaspirodecanediones

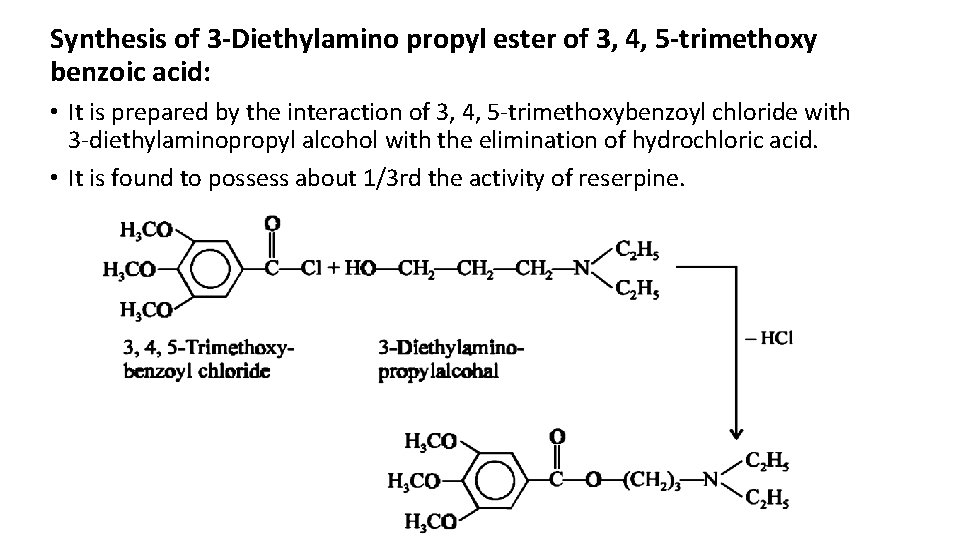
Reserpine and Related Alkaloids • The roots of Rauwolfia serpentina, a climbing shrub indigenous to India, and named after the German botanist Rauwolf, contains an alkaloid Reserpine which was reported to possess both tranquilizing and hypotensive properties. • Examples : Reserpine and Deserpidine. • Miller and Weinberg (1956) observed that even the simple tertiary amines having the trimethoxybenzoyl group exhibits the reserpine-like activity. • Example : 3 -Diethylamino propyl ester of 3, 4, 5 -trimethoxy benzoic acid

Synthesis of 3 -Diethylamino propyl ester of 3, 4, 5 -trimethoxy benzoic acid: • It is prepared by the interaction of 3, 4, 5 -trimethoxybenzoyl chloride with 3 -diethylaminopropyl alcohol with the elimination of hydrochloric acid. • It is found to possess about 1/3 rd the activity of reserpine.

Diphenylmethane Compounds • A number of diphenylmethane derivatives have been synthesized that exhibit antipsychotic activities. • A few such compounds are named : Pipradrol ; Captodiame ; Hydroxyzine ; Benactyzine ; • Pipradrol: • It is used for the treatment of functional fatigue and various types of depressions. The ‘drug’ exerts its action as a stimulant of the central nervous system

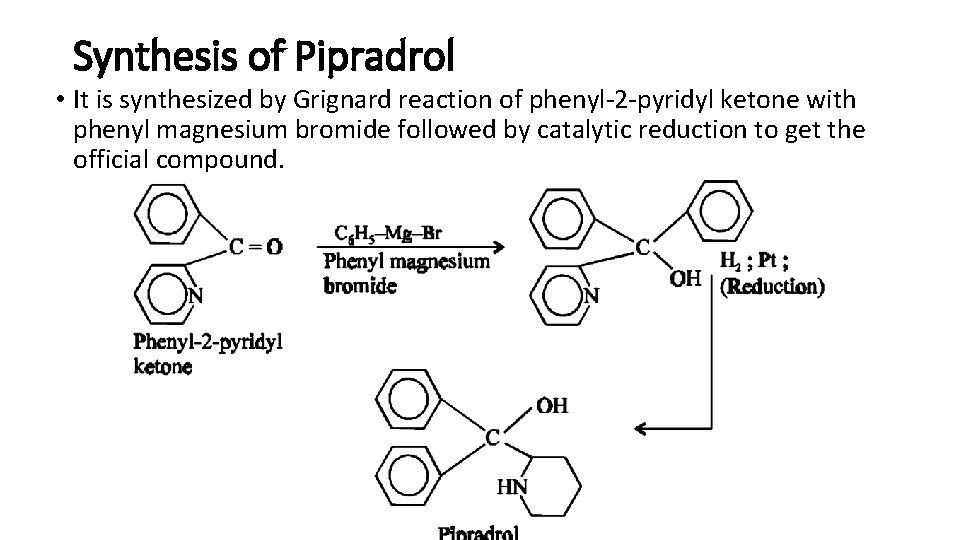
Synthesis of Pipradrol • It is synthesized by Grignard reaction of phenyl-2 -pyridyl ketone with phenyl magnesium bromide followed by catalytic reduction to get the official compound.

Phenothiazine Compounds • Chlorpromazine in the famous Rhone-Poulenc Laboratories in France in the year 1950 which was found to possess remarkable ameliorative effect on anxiety, agitation and psychoses. This ultimately led to the synthesis of a host of structural analogues of chlorpromazine that were found to be useful as antipsychotics. • A few typical examples from this class of compounds: Chlorpromazine, Perphenazine, Thioridazine.

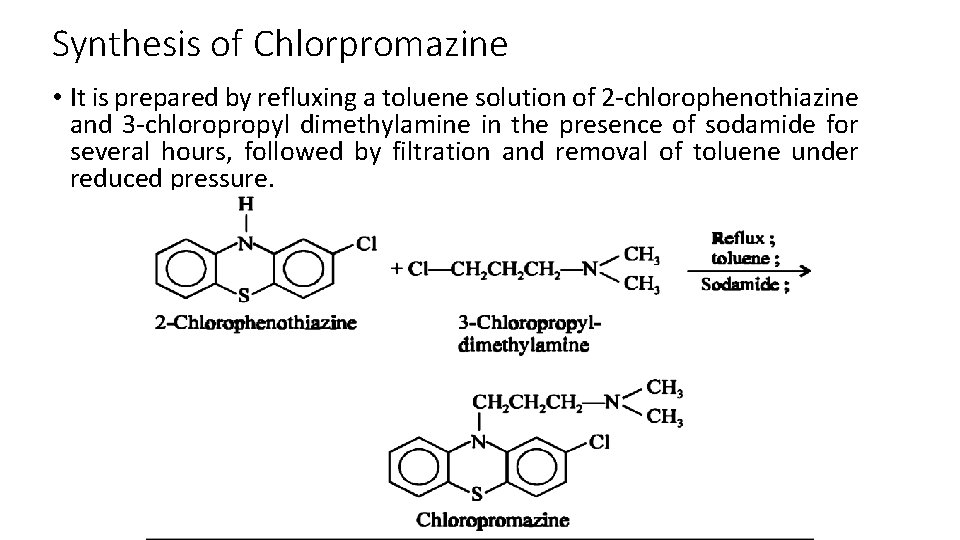
Synthesis of Chlorpromazine • It is prepared by refluxing a toluene solution of 2 -chlorophenothiazine and 3 -chloropropyl dimethylamine in the presence of sodamide for several hours, followed by filtration and removal of toluene under reduced pressure.

BARBITURATES Structure-Activity Relationships • Both hydrogen atoms at the 5 position of barbituric acid must be replaced. This may be because if one hydrogen is available at position 5, tautomerization to a highly acidic trihydroxypyrimidine (p. Ka ~4) can occur. • Beginning with lower alkyls, there is an increase in onset and a decrease in duration of action with increasing hydrocarbon content up to about seven to nine total carbon atoms substituted on the 5 position.

• Branched, cyclic or unsaturated chains in the 5 position generally produce a shorter duration of action. • Compounds with an alkyl group in the 1 or 3 position may have a shorter onset & duration of action. • Replacement of oxygen by sulfur on the 2 -carbon shortens the onset & duration of action. • Lipophilicity and an ability to penetrate the brain in the first case and an ability to penetrate liver microsomes in the second may be involved. • There is an inverse correlation between lipophilicity & the total number of carbon atoms substituted on the 5 position.

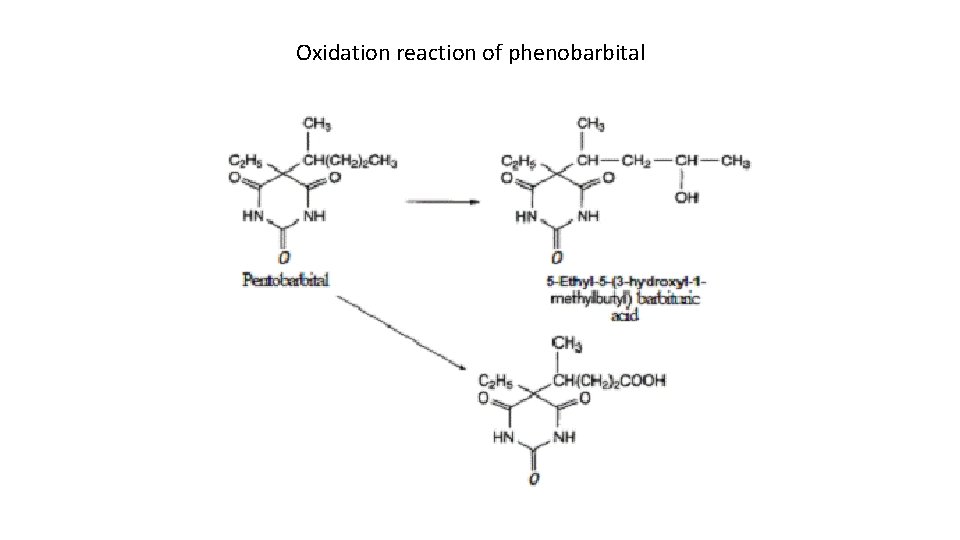
Metabolism of barbiturates: The principal site of metabolic inactivation is in the liver. In the metabolism, the lipophilic character of the barbiturates decreases, which in turn decreases the ability of the barbiturates to penetrate into the CNS. There are four primary metabolic processes that may take place. 1. Oxidation of substituents attached to C 5 is the most important pathway of metabolism for the barbiturates. The oxidative processes may yield alcohols, ketones, and carboxylic acids. For example, pentobarbital is oxidized to a hydroxy compound a carboxylic acid. 2. The oxidative process may also yield phenols. For example, phenobarbital is metabolized to β-hydroxyphenobarbital. The oxygenated metabolites (alcohols, phenols, ketones, and carboxylic acids) may be excreted in the urine in the free form or conjugated with glucuronic or sulfuric acid.

Oxidation reaction of phenobarbital

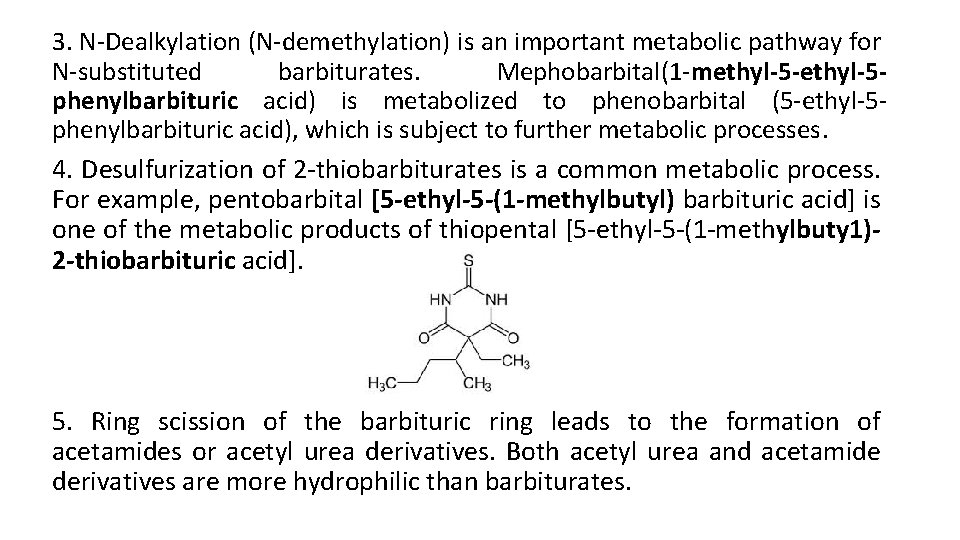
3. N-Dealkylation (N-demethylation) is an important metabolic pathway for N-substituted barbiturates. Mephobarbital(1 -methyl-5 -ethyl-5 phenylbarbituric acid) is metabolized to phenobarbital (5 -ethyl-5 phenylbarbituric acid), which is subject to further metabolic processes. 4. Desulfurization of 2 -thiobarbiturates is a common metabolic process. For example, pentobarbital [5 -ethyl-5 -(1 -methylbutyl) barbituric acid] is one of the metabolic products of thiopental [5 -ethyl-5 -(1 -methylbuty 1)2 -thiobarbituric acid]. 5. Ring scission of the barbituric ring leads to the formation of acetamides or acetyl urea derivatives. Both acetyl urea and acetamide derivatives are more hydrophilic than barbiturates.

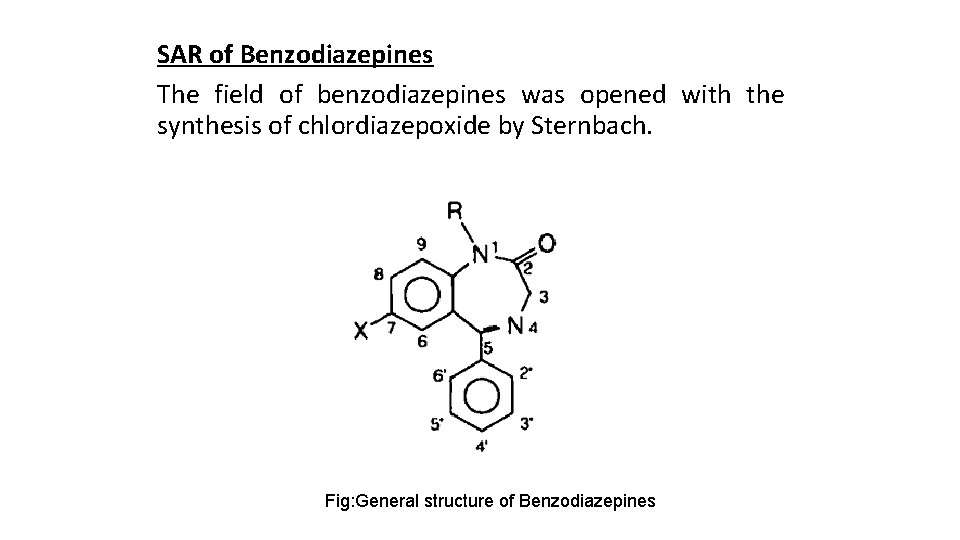
SAR of Benzodiazepines The field of benzodiazepines was opened with the synthesis of chlordiazepoxide by Sternbach. Fig: General structure of Benzodiazepines

• An electronegative substituent at position 7 in required for activity, and the more electronegative it is the higher the activity. • Positions 6, 8, and 9 should not be substituted. • A phenyl at position 5 promotes activity. →If this phenyl group is ortho (2') or diortho (2'o') substituted with electron-attracting substituents, activity is increased. →On the other hand para substitution decreases activity greatly. • Saturation of the 4, 5 double bond or a shift of it to the 3, 4 position decreases activity.

• Alkyl substitution at the 3 position decreases activity: substitution with a hydroxy does not. • The presence or absence of the 3 -hydroxyl is important pharmacokinetically. →Compounds without the hydroxyl are nonpolar, have long half-lives, and undergo hepatic oxidation. →Compounds with the hydroxyl are much more polar and are readily converted to the excreted glucuronide. • The 2 -carbonyl function is optimal for activity, as is the nitrogen atom at position l. • The N-substituent should be small.

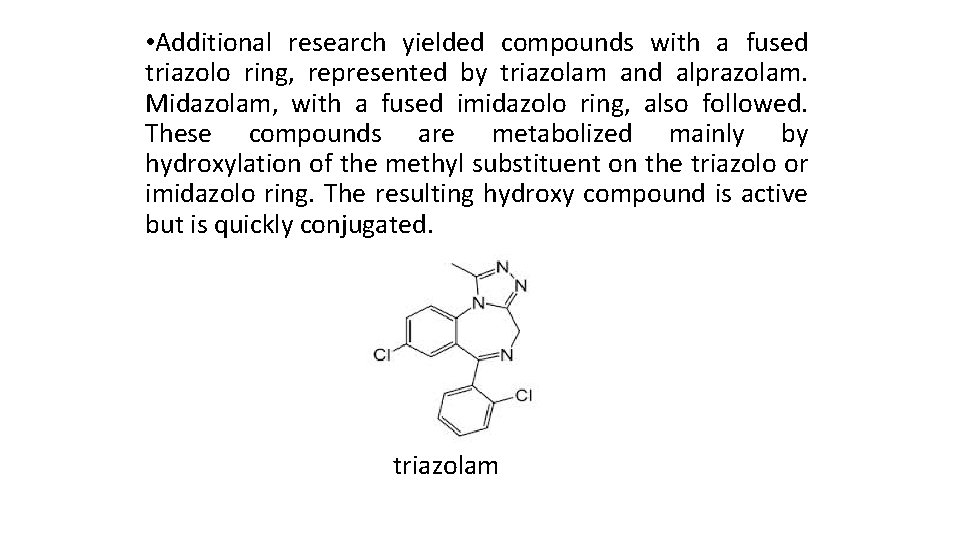
• Additional research yielded compounds with a fused triazolo ring, represented by triazolam and alprazolam. Midazolam, with a fused imidazolo ring, also followed. These compounds are metabolized mainly by hydroxylation of the methyl substituent on the triazolo or imidazolo ring. The resulting hydroxy compound is active but is quickly conjugated. triazolam

Metabolism of Benzodiazepines • Benzodiazepines relatively rapidly cross the blood-brain barrier and equilibrate with brain tissue. • The two principal pathways of the BDZ biotransformation involve hepatic microsomal oxidation, N-dealkylation or aliphatic hydroxylation and glucuronide conjugation. • The metabolites are excreted mainly by the kidney. Many hydroxylated metabolites of BDZs are pharmacologically active, some with long half-lives.

• A benzodiazepine can be placed into one of three groups by its elimination half-life. • Short-acting compounds have a median half-life of 1– 12 hours. They have few residual effects, withdrawal symptoms. Examples are brotizolam, midazolam, and triazolam. • Intermediate-acting compounds have a median half-life of 12– 40 hours. Rebound insomnia can commonly occur upon discontinuation. Examples are alprazolam, estazolam, flunitrazepam, clonazepam, lormetazepam, lorazepam, nitrazepam, and temazepam. • Long-acting compounds have a half-life of 40– 250 hours. They have a risk of accumulation in the elderly and in individuals with severely impaired liver function, but they have a reduced severity of rebound effects and withdrawal. Examples are diazepam, clorazepate, chlordiazepoxide, and flurazepam.