Tracking Protocol Development Timelines Steve Friedman MHSA Chief








- Slides: 13

Tracking Protocol Development Timelines Steve Friedman, MHSA Chief, Clinical Trials Operations and Informatics Branch

Protocol Timelines Tracking - Current • CTEP’s PIO has been tracking and reporting protocol activity via: – Bi-monthly reports to Cooperative Groups – Queries to PIO – Protocol Complete Sheets (NCI internal use) • Majority of milestones already captured by CTEP Enterprise System (remainder are being added) • Need for enhanced tracking mechanisms precipitated by OEWG recommendations

Protocol Timelines Tracking - Future • As of Q 3 2010, sites can access secure web site to retrieve timelines tracking reports • Between Q 3 2010 and Q 1 2011, CTEP will solicit feedback on reports and modify • Reports will be finalized prior to the start of the live period of OEWG recommended timelines • Benefit of new tools include: – Dynamic update to information – Multiple views – Role based access

CTEP Secure Website – Access/Login Click here to access the secure website Users with IAM accounts with the following roles on protocols will be able to access and view their protocols • Principal Investigator • Site Coordinator • Investigator • Mail to Contact • Primary CDUS Contact • Secondary CDUS Contact • Grant Investigator • Grant PI

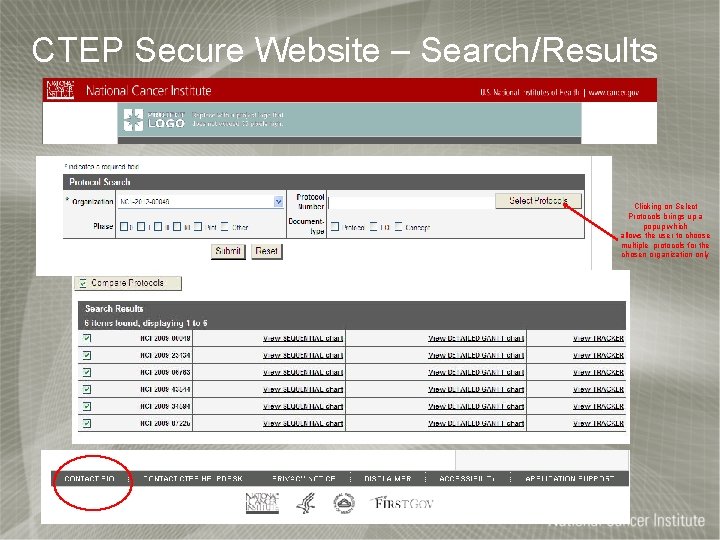
CTEP Secure Website – Search/Results Clicking on Select Protocols brings up a popup which allows the user to choose multiple protocols for the chosen organization only

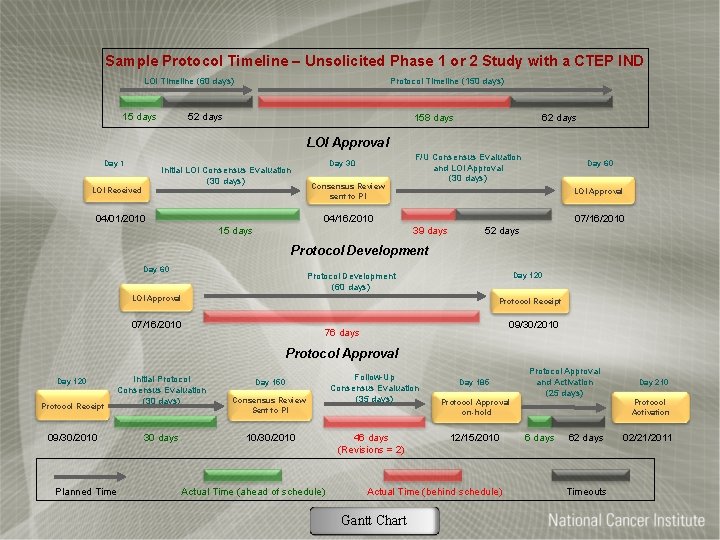
Sample Protocol Timeline – Unsolicited Phase 1 or 2 Study with a CTEP IND LOI Timeline (60 days) 15 days Protocol Timeline (150 days) 52 days 158 days 62 days LOI Approval Day 1 Initial LOI Consensus Evaluation (30 days) LOI Received 04/01/2010 Day 30 Consensus Review sent to PI F/U Consensus Evaluation and LOI Approval (30 days) Day 60 LOI Approval 04/16/2010 15 days 07/16/2010 39 days 52 days Protocol Development Day 60 Day 120 Protocol Development (60 days) LOI Approval Protocol Receipt 07/16/2010 09/30/2010 76 days Protocol Approval Day 120 Protocol Receipt 09/30/2010 Planned Time Initial Protocol Consensus Evaluation (30 days) 30 days Day 150 Consensus Review Sent to PI 10/30/2010 Actual Time (ahead of schedule) Follow-Up Consensus Evaluation (35 days) 46 days (Revisions = 2) Day 185 Protocol Approval on-hold 12/15/2010 Actual Time (behind schedule) Gantt Chart Protocol Approval and Activation (25 days) Day 210 Protocol Activation 6 days 62 days Timeouts 02/21/2011

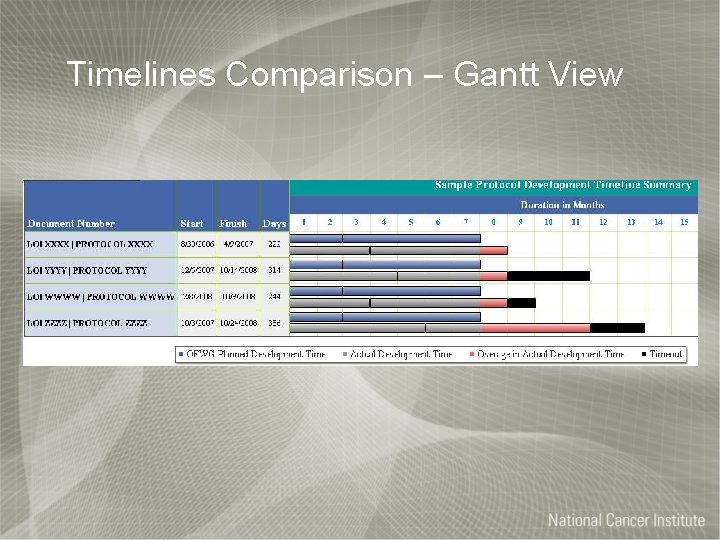
Timelines Comparison – Gantt View

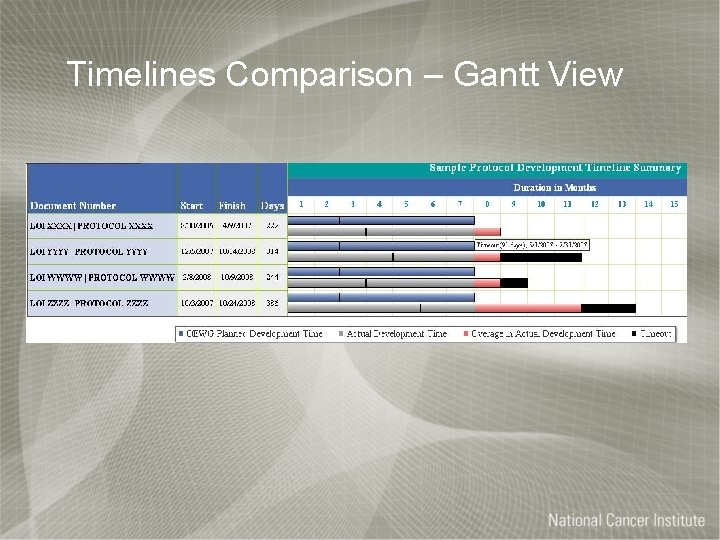
Timelines Comparison – Gantt View

Protocol Tracker

Protocol Timelines – Detailed Gantt Sequential Chart

Protocol Timelines – Detailed Gantt Sequential Chart

Protocol Timelines Tracking - Interim • CTEP will develop set of spreadsheets that will contain key information: – – – PI name Org name Protocol # Completed milestones/stages Duration of each completed milestone/stage • Spreadsheets will be disseminated monthly until secure site is ready Post-meeting update: The Timelines Report website can now be found via the “Secure Access” tab in the upper right hand corner of the CTEP website (http: //ctep. cancer. gov).
