Trace Element Behavior during Crystallization Lecture 27 Trace

Trace Element Behavior during Crystallization Lecture 27
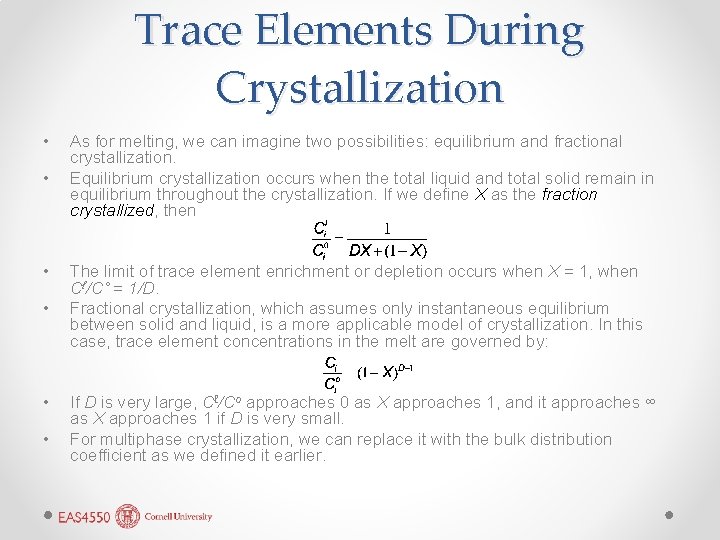
Trace Elements During Crystallization • • • As for melting, we can imagine two possibilities: equilibrium and fractional crystallization. Equilibrium crystallization occurs when the total liquid and total solid remain in equilibrium throughout the crystallization. If we define X as the fraction crystallized, then The limit of trace element enrichment or depletion occurs when X = 1, when Cℓ/C˚ = 1/D. Fractional crystallization, which assumes only instantaneous equilibrium between solid and liquid, is a more applicable model of crystallization. In this case, trace element concentrations in the melt are governed by: If D is very large, Cℓ/Co approaches 0 as X approaches 1, and it approaches ∞ as X approaches 1 if D is very small. For multiphase crystallization, we can replace it with the bulk distribution coefficient as we defined it earlier.
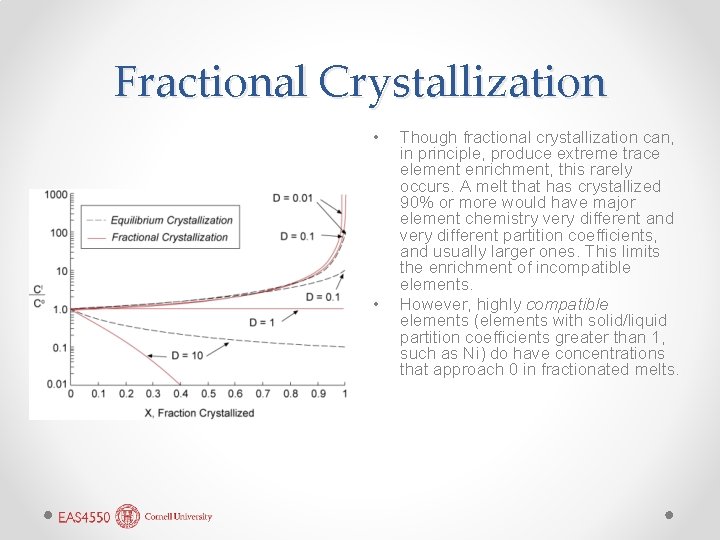
Fractional Crystallization • • Though fractional crystallization can, in principle, produce extreme trace element enrichment, this rarely occurs. A melt that has crystallized 90% or more would have major element chemistry very different and very different partition coefficients, and usually larger ones. This limits the enrichment of incompatible elements. However, highly compatible elements (elements with solid/liquid partition coefficients greater than 1, such as Ni) do have concentrations that approach 0 in fractionated melts.
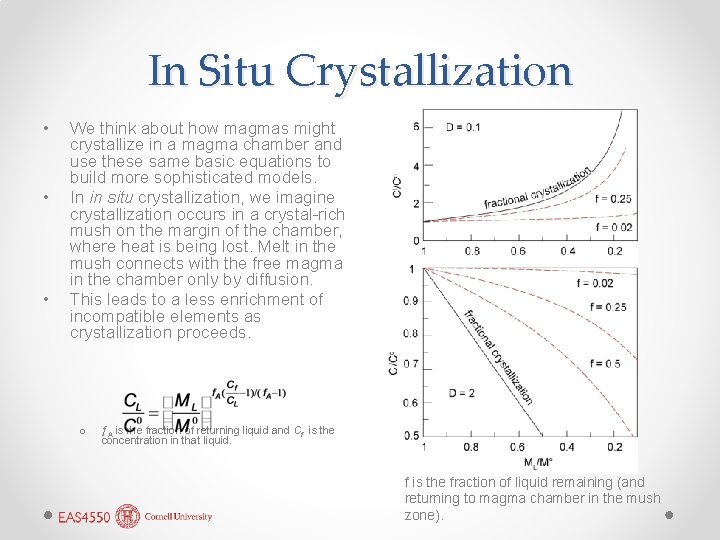
In Situ Crystallization • • • We think about how magmas might crystallize in a magma chamber and use these same basic equations to build more sophisticated models. In in situ crystallization, we imagine crystallization occurs in a crystal-rich mush on the margin of the chamber, where heat is being lost. Melt in the mush connects with the free magma in the chamber only by diffusion. This leads to a less enrichment of incompatible elements as crystallization proceeds. o ƒA is rhe fraction of returning liquid and Cf is the concentration in that liquid. f is the fraction of liquid remaining (and returning to magma chamber in the mush zone).
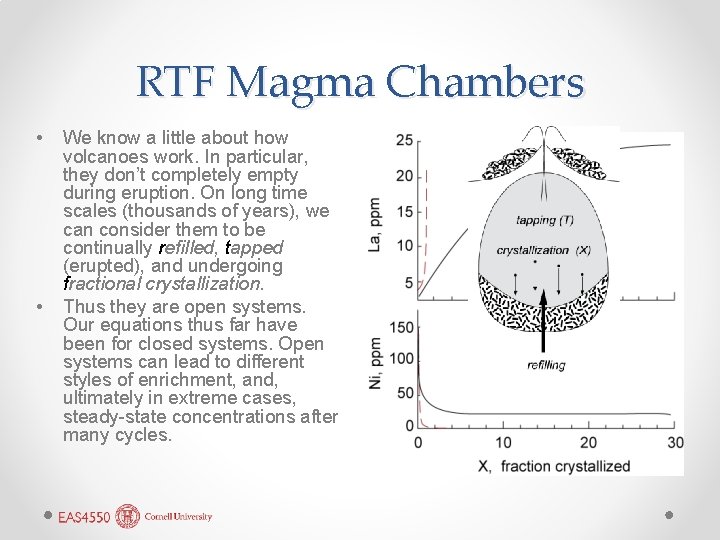
RTF Magma Chambers • • We know a little about how volcanoes work. In particular, they don’t completely empty during eruption. On long time scales (thousands of years), we can consider them to be continually refilled, tapped (erupted), and undergoing fractional crystallization. Thus they are open systems. Our equations thus far have been for closed systems. Open systems can lead to different styles of enrichment, and, ultimately in extreme cases, steady-state concentrations after many cycles.
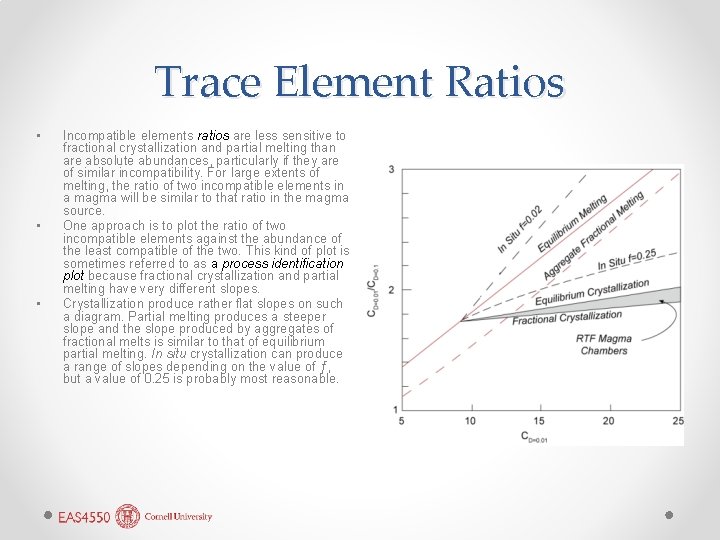
Trace Element Ratios • • • Incompatible elements ratios are less sensitive to fractional crystallization and partial melting than are absolute abundances, particularly if they are of similar incompatibility. For large extents of melting, the ratio of two incompatible elements in a magma will be similar to that ratio in the magma source. One approach is to plot the ratio of two incompatible elements against the abundance of the least compatible of the two. This kind of plot is sometimes referred to as a process identification plot because fractional crystallization and partial melting have very different slopes. Crystallization produce rather flat slopes on such a diagram. Partial melting produces a steeper slope and the slope produced by aggregates of fractional melts is similar to that of equilibrium partial melting. In situ crystallization can produce a range of slopes depending on the value of ƒ, but a value of 0. 25 is probably most reasonable.
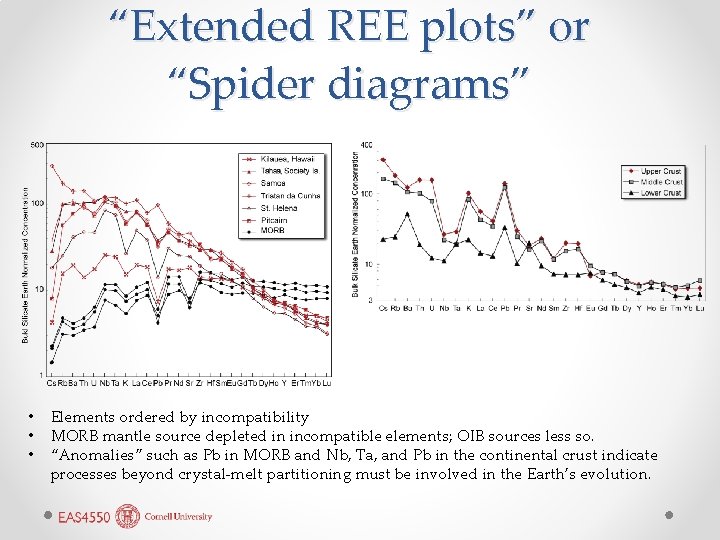
“Extended REE plots” or “Spider diagrams” • • • Elements ordered by incompatibility MORB mantle source depleted in incompatible elements; OIB sources less so. “Anomalies” such as Pb in MORB and Nb, Ta, and Pb in the continental crust indicate processes beyond crystal-melt partitioning must be involved in the Earth’s evolution.

Summary • Incompatible element abundances are strong functions of degree of melting (more so for fractional), compatible element abundances are only weakly dependent on extent of melting. o • Moderate amounts of fractional crystallization do not have dramatic effects on incompatible element concentrations. Concentrations of highly compatible elements dramatically decrease with extent of fractional crystallization. o • • The RTF model does have significantly greater effects on incompatible element concentrations than simpler models, however. This all works out nicely: compatible elements are good qualitative indicators of the extent of fractional crystallization and incompatible elements are good indicators of the degree of melting. Both geochemical and experimental evidence indicates that alkali basalts and are the products of lower degrees of melting than tholeiites such as MORB, which are generally produced by ~8 to 15% average extent of melting. o • Two simplifications are important for partial melting. When D ≪ F, the enrichment is 1/F for batch melting. If D is large (i. e. , D ≫ F), the depletion of the element in the melt is rather insensitive to F. when In either F approaches case, 0, the maximum enrichment or depletion is 1/D. Highly undersaturated rocks such as nephelinites are probably produced by the smallest degrees of melting (1% or less). Incompatible element ratios are less sensitive to fractional crystallization and partial melting than are absolute abundances, particularly if they are of similar incompatibility. o For relatively large extents of melting, the ratio of two incompatible elements in a magma will be similar to that ratio in the magma source.

Take Away • Trace element variations help us understand reconstruct magmatic process such as partial melting and fractional crystallization. • Patterns of trace elements also reflect the history of magma sources: o For example, LREE and incompatible element depletion of MORB o Incompatible element enrichment of oceanic island basalts (OIB) o Ta-Nb depletion of typical continental crust • Radiogenic Isotope ratios reflect ancient trace element fractionations and are functions of time –allowing us to begin to reconstruct histories.
- Slides: 9