Toxins Unit Investigation III Precipitating Toxins Lesson 1


















































- Slides: 96

Toxins Unit Investigation III: Precipitating Toxins Lesson 1: Solid Evidence Lesson 2: I’ve Got My Ion You Lesson 3: Sticks and Stones Lesson 4: Blockhead Lesson 5: Mass Appeal Lesson 6: Get the Lead Out Lesson 7: Grammies

Toxins Unit – Investigation III Lesson 1: Solid Evidence

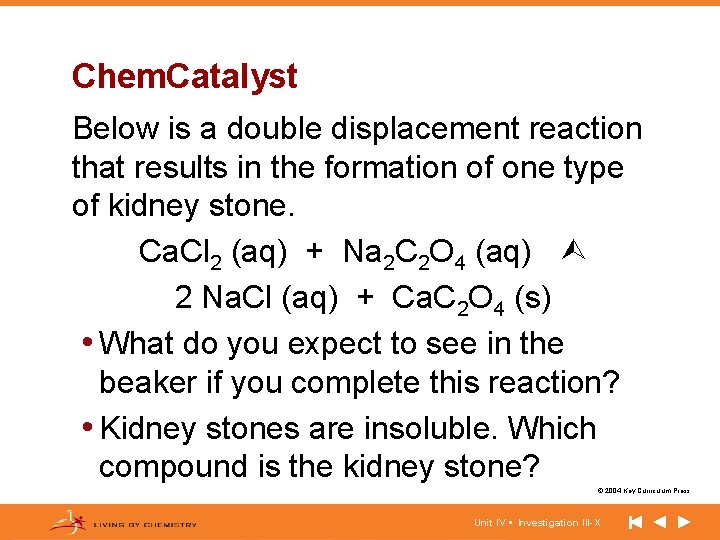
Chem. Catalyst Below is a double displacement reaction that results in the formation of one type of kidney stone. Ca. Cl 2 (aq) + Na 2 C 2 O 4 (aq) 2 Na. Cl (aq) + Ca. C 2 O 4 (s) • What do you expect to see in the beaker if you complete this reaction? • Kidney stones are insoluble. Which compound is the kidney stone? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

The Big Question • What is a precipitation reaction and how can you determine whether a precipitate will form? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

You will be able to: Use a solubility table to predict whether a particular chemical reaction will be a precipitation reaction. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

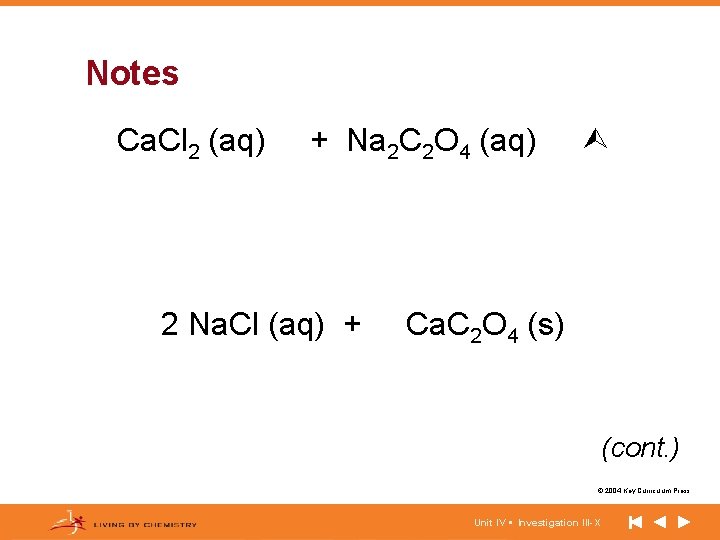
Notes Ca. Cl 2 (aq) + Na 2 C 2 O 4 (aq) 2 Na. Cl (aq) + Ca. C 2 O 4 (s) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes (cont. ) • Precipitate: A substance of a different phase that separates out of a solution. • A reaction in which a precipitate forms is called a precipitation reaction. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

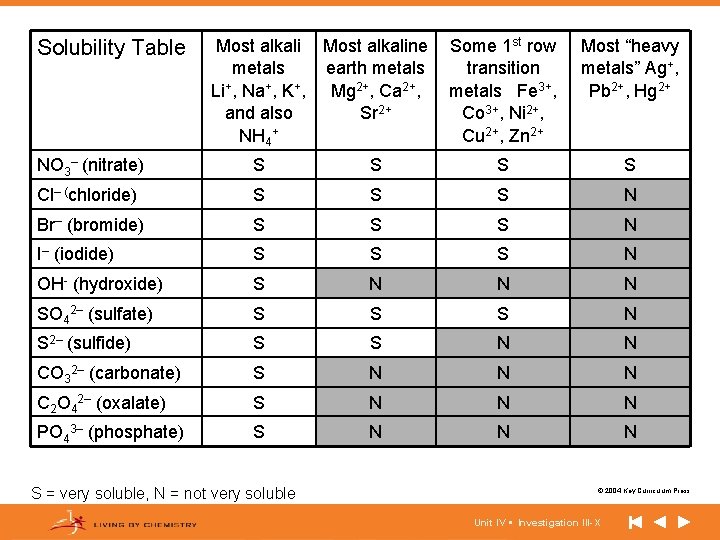
Solubility Table Most alkaline metals earth metals Li+, Na+, K+, Mg 2+, Ca 2+, and also Sr 2+ NH 4+ Some 1 st row transition metals Fe 3+, Co 3+, Ni 2+, Cu 2+, Zn 2+ Most “heavy metals” Ag+, Pb 2+, Hg 2+ NO 3– (nitrate) S S Cl– (chloride) S S S N Br– (bromide) S S S N I– (iodide) S S S N OH- (hydroxide) S N N N SO 42– (sulfate) S S S N S 2– (sulfide) S S N N CO 32– (carbonate) S N N N C 2 O 42– (oxalate) S N N N PO 43– (phosphate) S N N N S = very soluble, N = not very soluble © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Activity Purpose: In this experiment, you will predict the solubility of various ionic solids and then test your predictions. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Making Sense • Write three solubility rules. (Example: Alkali metal salts tend to be soluble. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes If we examine the solubility table and the results of the experiment, certain patterns emerge: • Most Group I and NH 4+ salts are soluble. • Most nitrates, NO 3–, salts are soluble. • Most chlorides, bromides, and iodides are soluble (except for Ag+, Pb 2+, Hg 2+) • Most carbonates, oxalates, and phosphates are insoluble. • Most salts of heavy metals are insoluble. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes (cont. ) The pros and cons of the solubility of toxins: • (Con) Things that are soluble get into the water-based systems of our bodies more easily. Once dissolved in the bloodstream they may interact in negative ways with our bodies. • (Pro) Things that are soluble are easier to filter out of the body using our natural filtration systems. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes (cont. ) • (Con) Things that are insoluble may build up inside the body, causing blockages. • (Pro) Things that are insoluble may pass right through the body without causing harm. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

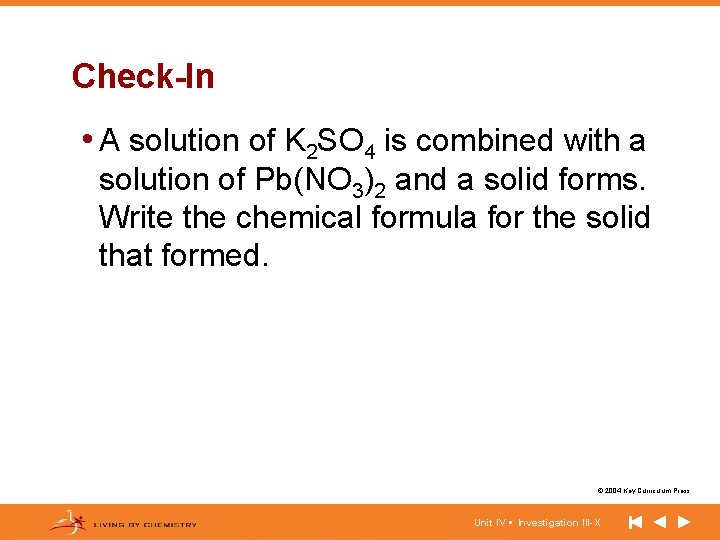
Check-In • A solution of K 2 SO 4 is combined with a solution of Pb(NO 3)2 and a solid forms. Write the chemical formula for the solid that formed. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Wrap-Up • A precipitate is a solid produced in a chemical reaction between two solutions. • Most alkali metal compounds and most metal nitrates are soluble. Halides tend to be soluble, except for heavy metal halides. Heavy metal compounds tend to be insoluble. • Solubility can interact with the human body in either positive or negative ways. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Toxins Unit – Investigation III Lesson 2: I’ve Got My Ion You

Chem. Catalyst • Use your results from the experiments you did yesterday to write a balanced chemical reaction to describe what happens when you mix Na 2 CO 3 (aq), sodium carbonate, with Mg(NO 3)2 (aq), magnesium nitrate. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

The Big Question • What is the role of ions in the precipitation reaction process? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

You will be able to: Predict the products of precipitation reactions and write balanced chemical equations that represent the precipitation reaction process. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Activity Purpose: This activity provides practice with equations involving ionic compounds. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

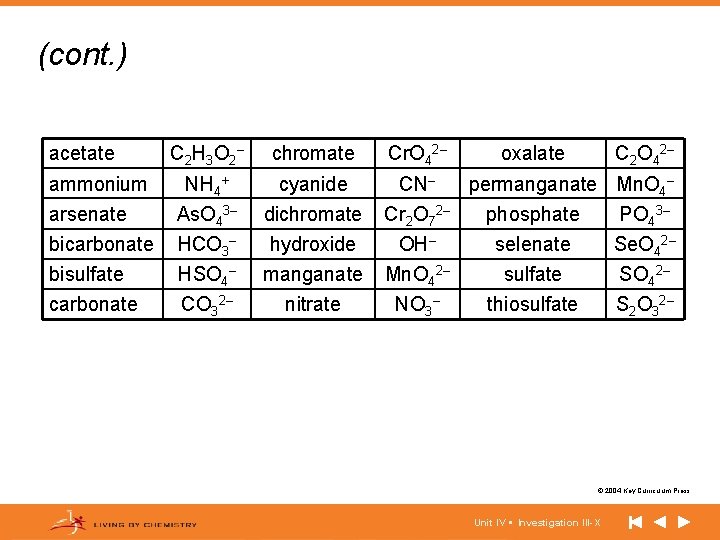
(cont. ) acetate C 2 H 3 O 2 – chromate Cr. O 42– NH 4+ cyanide CN– arsenate As. O 43– dichromate Cr 2 O 72– phosphate PO 43– bicarbonate HCO 3– hydroxide OH– selenate Se. O 42– bisulfate HSO 4– manganate Mn. O 42– sulfate SO 42– carbonate CO 32– nitrate NO 3– thiosulfate S 2 O 32– ammonium oxalate C 2 O 42– permanganate Mn. O 4– © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Making Sense • How can you predict whether you will see a solid when you dissolve a salt in water? • What patterns do you notice between charges and solubility, in question 5? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

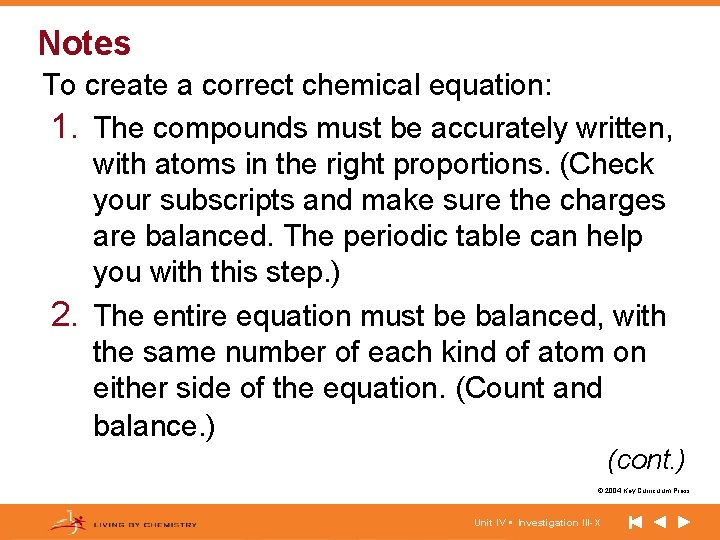
Notes To create a correct chemical equation: 1. The compounds must be accurately written, with atoms in the right proportions. (Check your subscripts and make sure the charges are balanced. The periodic table can help you with this step. ) 2. The entire equation must be balanced, with the same number of each kind of atom on either side of the equation. (Count and balance. ) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

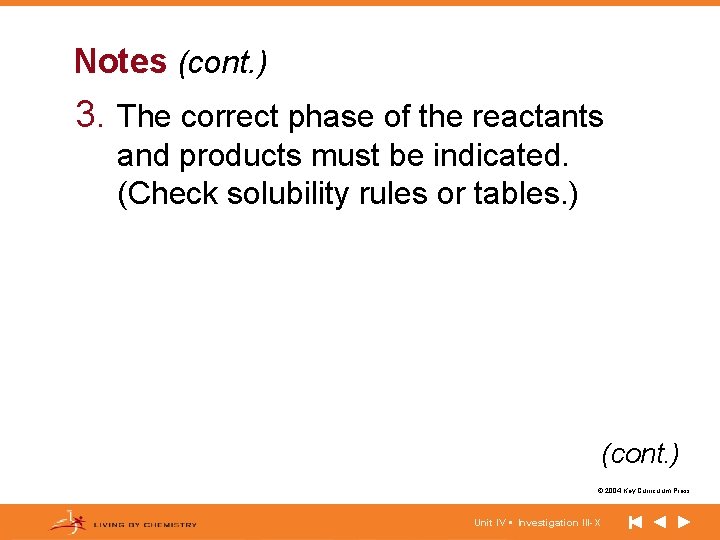
Notes (cont. ) 3. The correct phase of the reactants and products must be indicated. (Check solubility rules or tables. ) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

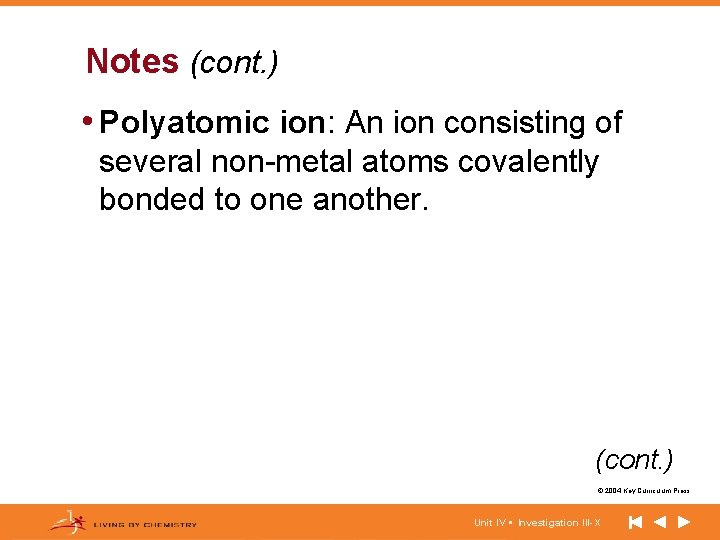
Notes (cont. ) • Polyatomic ion: An ion consisting of several non-metal atoms covalently bonded to one another. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

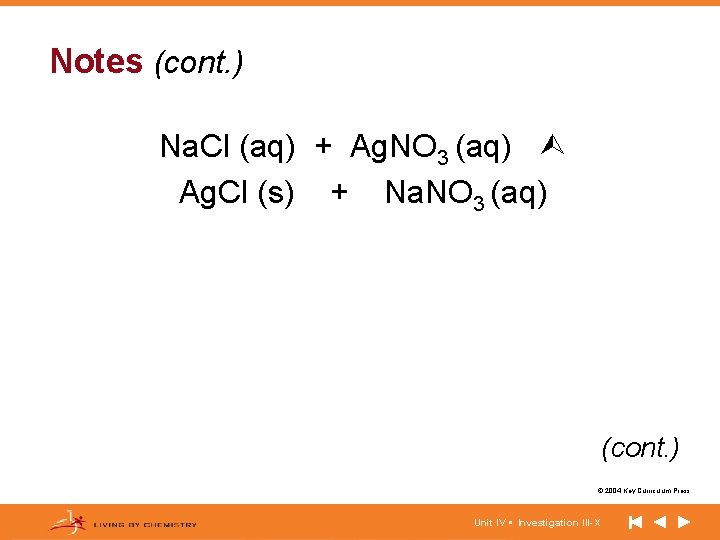
Notes (cont. ) Na. Cl (aq) + Ag. NO 3 (aq) Ag. Cl (s) + Na. NO 3 (aq) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

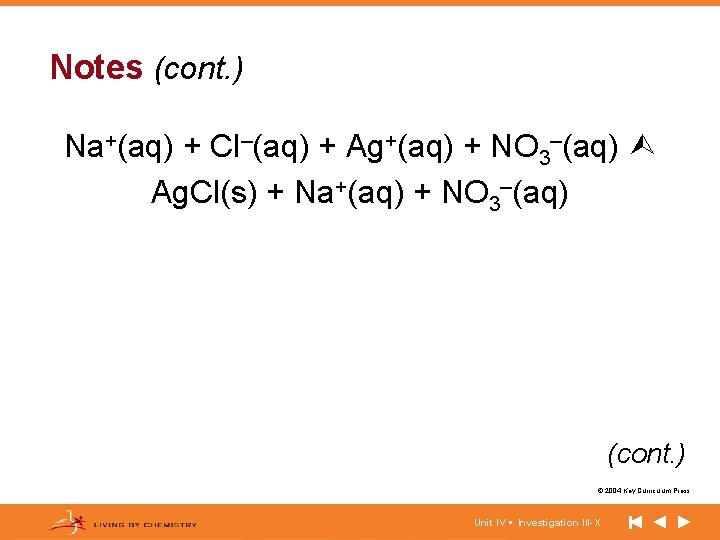
Notes (cont. ) Na+(aq) + Cl–(aq) + Ag+(aq) + NO 3–(aq) Ag. Cl(s) + Na+(aq) + NO 3–(aq) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

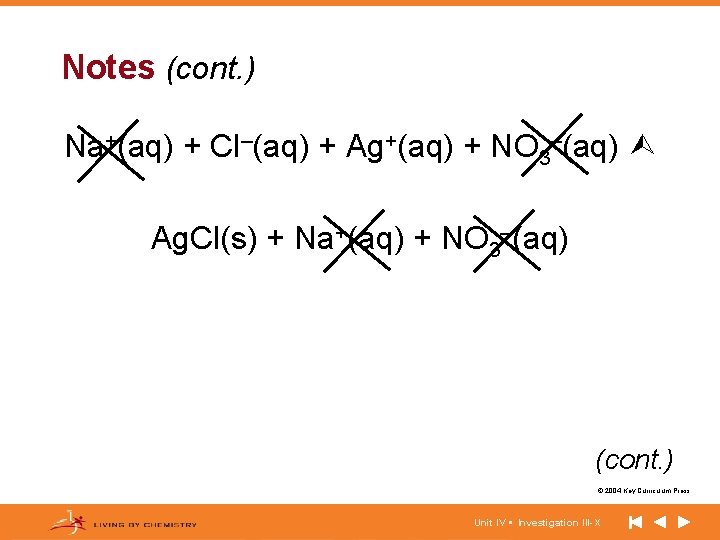
Notes (cont. ) Na+(aq) + Cl–(aq) + Ag+(aq) + NO 3–(aq) Ag. Cl(s) + Na+(aq) + NO 3–(aq) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

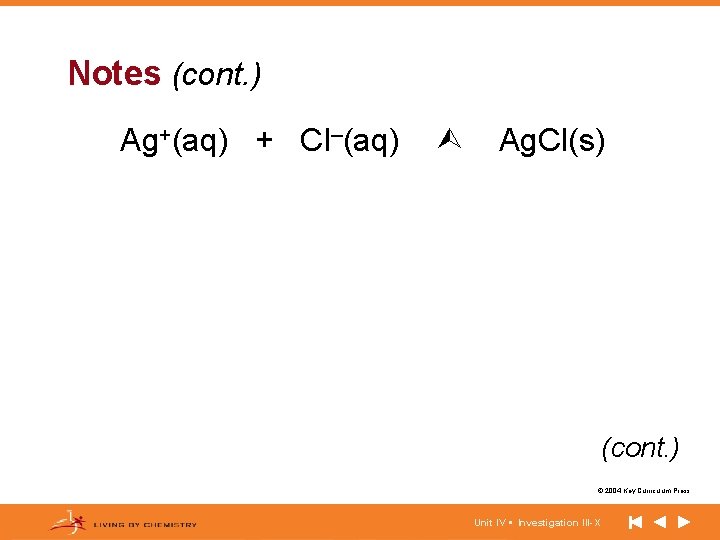
Notes (cont. ) Ag+(aq) + Cl–(aq) Ag. Cl(s) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes (cont. ) • Overall ionic equation: Chemical equation written with the dissolved salts as aqueous ions. • Net ionic equation: Equation written with only those species that participate in the reaction. • Spectator ions: Ions that do not participate in the reaction. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Check-In • Write a balanced chemical equation describing what happens when you mix sodium chromate and calcium nitrate. (The chromate ion is Cr. O 42–) • Predict whether each compound should be labeled as (aq) or (s). © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Wrap-Up • The specific charges on ions can be deduced from the periodic table. • Some non-metal atoms remain covalently bonded as polyatomic ions. • If the charges of the cation and anion are low (e. g. , +1 and – 1), the compound tends to be soluble. If the charges are higher (e. g. , +2 and – 2 or – 3), the compounds tend to be insoluble. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Toxins Unit – Investigation III Lesson 3: Sticks and Stones

Chem. Catalyst Oxalate compounds are a common part of our daily diet. Some examples of foods that are high in oxalate are chocolate, eggplant, graham crackers, and strawberries. Too much oxalate in the body can cause kidney stones. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

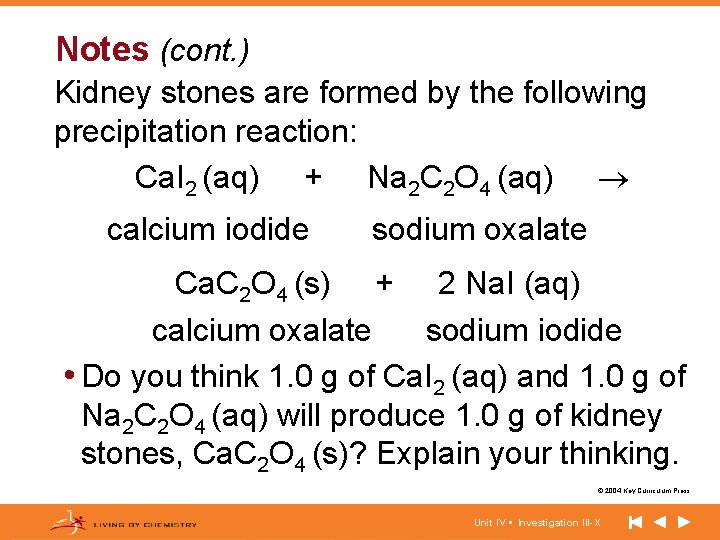
Notes (cont. ) Kidney stones are formed by the following precipitation reaction: Ca. I 2 (aq) + Na 2 C 2 O 4 (aq) calcium iodide sodium oxalate Ca. C 2 O 4 (s) + 2 Na. I (aq) calcium oxalate sodium iodide • Do you think 1. 0 g of Ca. I 2 (aq) and 1. 0 g of Na 2 C 2 O 4 (aq) will produce 1. 0 g of kidney stones, Ca. C 2 O 4 (s)? Explain your thinking. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

The Big Question • What do the coefficients in a balanced chemical reaction mean, and how do they relate to real-world observations? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

You will be able to: Experimentally find the highest-yielding ratios of reactants in a precipitation reaction and relate your results to the balanced chemical equation for the reaction. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Activity Purpose: You will determine what ratio of reactants gives the maximum amount of products. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

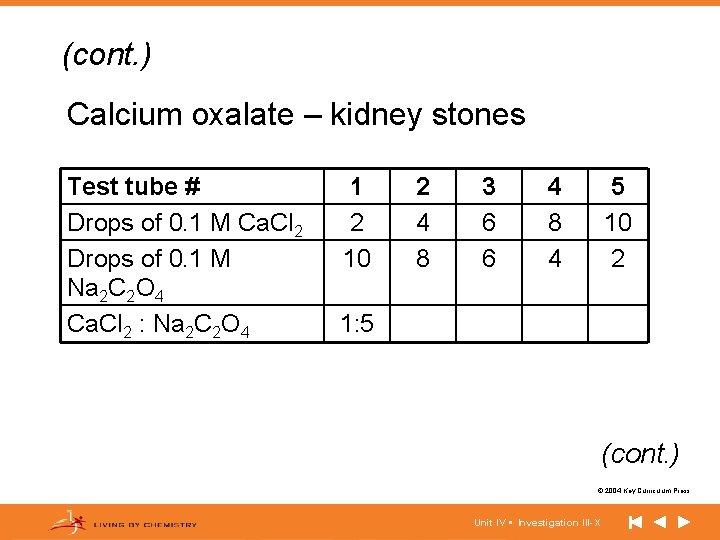
(cont. ) Calcium oxalate – kidney stones Test tube # Drops of 0. 1 M Ca. Cl 2 Drops of 0. 1 M Na 2 C 2 O 4 Ca. Cl 2 : Na 2 C 2 O 4 1 2 10 2 4 8 3 6 6 4 8 4 5 10 2 1: 5 (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

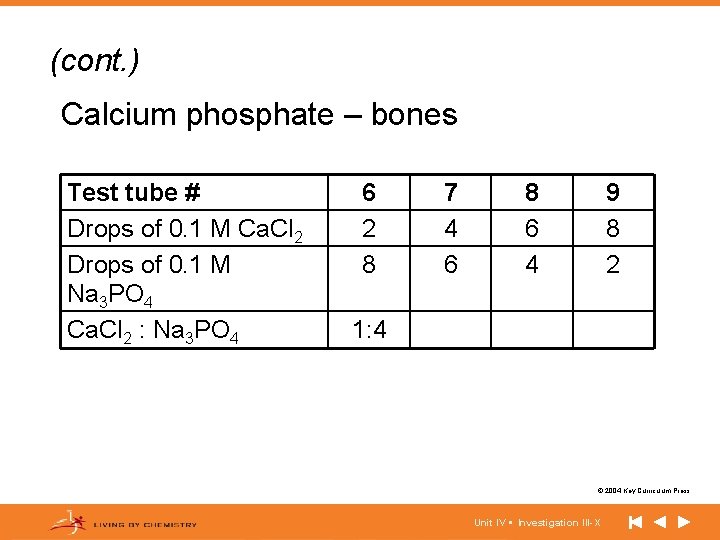
(cont. ) Calcium phosphate – bones Test tube # Drops of 0. 1 M Ca. Cl 2 Drops of 0. 1 M Na 3 PO 4 Ca. Cl 2 : Na 3 PO 4 6 2 8 7 4 6 8 6 4 9 8 2 1: 4 © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Making Sense • Explain how you can use the coefficients in the balanced chemical equation to determine the ratio of reactants that will produce the maximum amount of product. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes • A formula unit is the chemical formula that describes a substance that is not molecular. It is the simplest ratio of atoms found in the substance. For example, Ca. Cl 2 represents one formula unit of calcium chloride. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

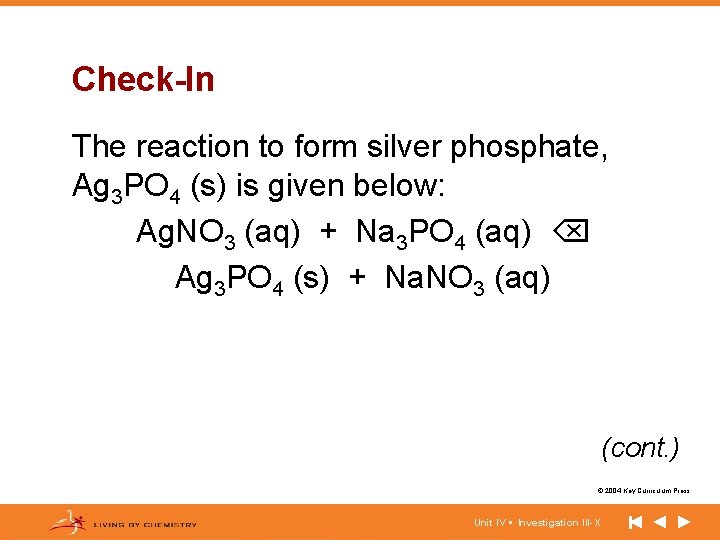
Check-In The reaction to form silver phosphate, Ag 3 PO 4 (s) is given below: Ag. NO 3 (aq) + Na 3 PO 4 (aq) Ag 3 PO 4 (s) + Na. NO 3 (aq) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

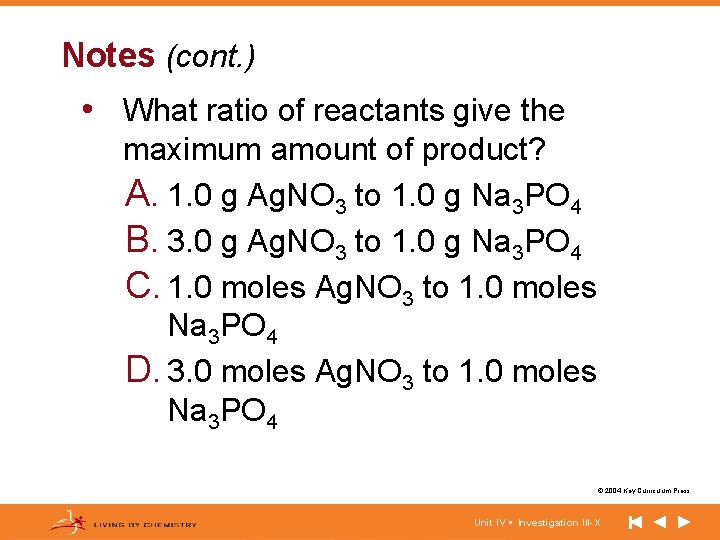
Notes (cont. ) • What ratio of reactants give the maximum amount of product? A. 1. 0 g Ag. NO 3 to 1. 0 g Na 3 PO 4 B. 3. 0 g Ag. NO 3 to 1. 0 g Na 3 PO 4 C. 1. 0 moles Ag. NO 3 to 1. 0 moles Na 3 PO 4 D. 3. 0 moles Ag. NO 3 to 1. 0 moles Na 3 PO 4 © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Wrap-Up • The coefficients found in chemical equations stand for counting units such as number of molecules, number of moles, etc. • Coefficients in chemical equations represent the ratio in which reactants combine and products form. • Mass and volume amounts cannot be substituted for coefficients. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Toxins Unit – Investigation III Lesson 4: Blockhead

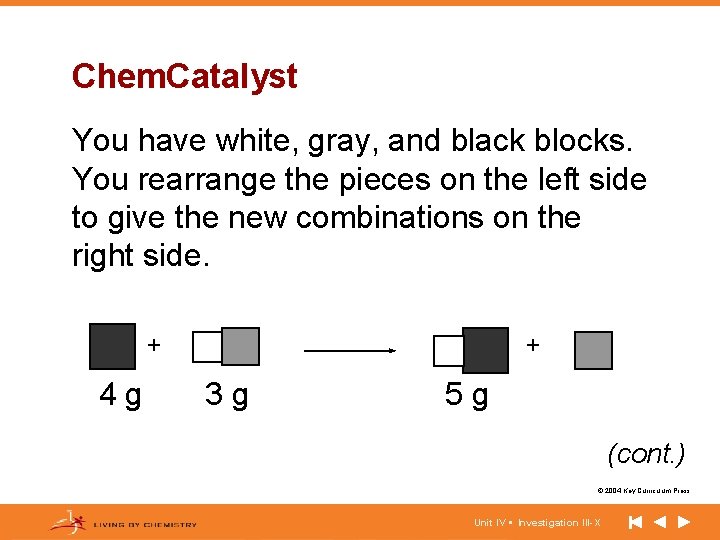
Chem. Catalyst You have white, gray, and black blocks. You rearrange the pieces on the left side to give the new combinations on the right side. + 4 g + 3 g 5 g (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes (cont. ) • How much does the gray block weigh? • If you have 40 g of black blocks and 30 g of white-gray pieces, how many white-black blocks can you make? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

The Big Question • How does the mole concept help you predict the amount of each product in a particular reaction? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

You will be able to: Calculate the quantity of a product formed in a reaction from a given quantity of starting reactants. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Activity Purpose: You will relate grams of reactants and grams of products using moles as an intermediary. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

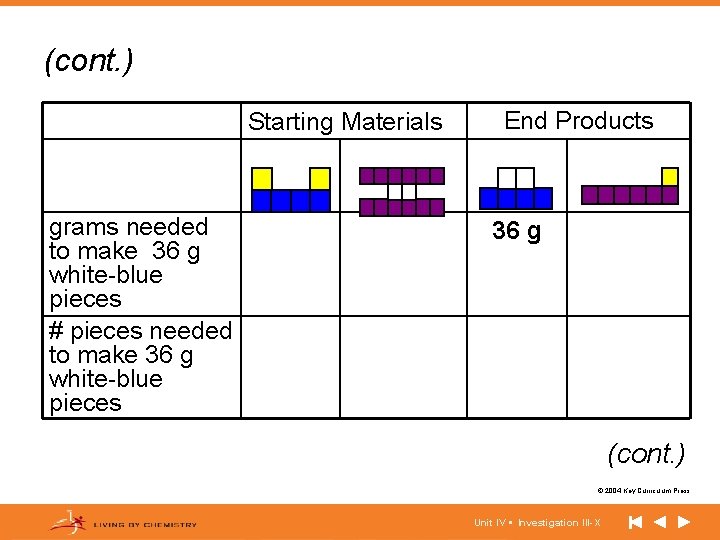
(cont. ) Starting Materials grams needed to make 36 g white-blue pieces # pieces needed to make 36 g white-blue pieces End Products 36 g (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

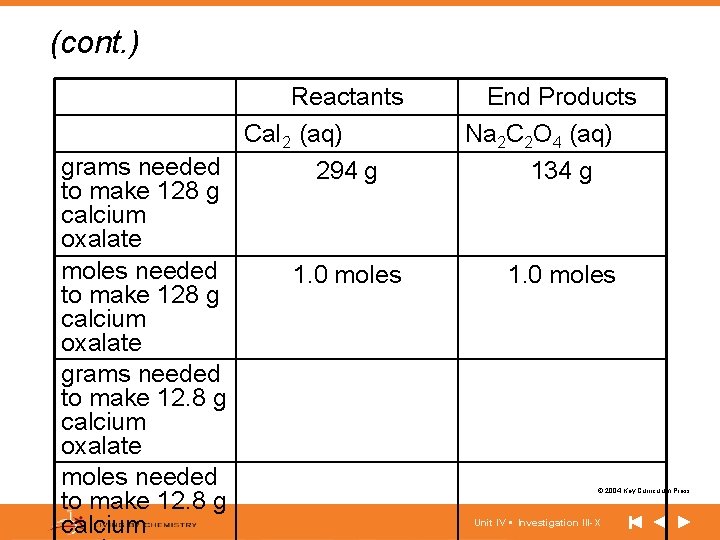
(cont. ) Reactants Ca. I 2 (aq) grams needed 294 g to make 128 g calcium oxalate moles needed 1. 0 moles to make 128 g calcium oxalate grams needed to make 12. 8 g calcium oxalate moles needed to make 12. 8 g calcium End Products Na 2 C 2 O 4 (aq) 134 g 1. 0 moles © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Making Sense • Explain how you determined the number of grams of white-purple pieces you needed to make approximately 36 grams of white-blue pieces. Explain how you determined the number of grams of Na 2 C 2 O 4 you need to make 12. 8 g of Ca. C 2 O 4. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

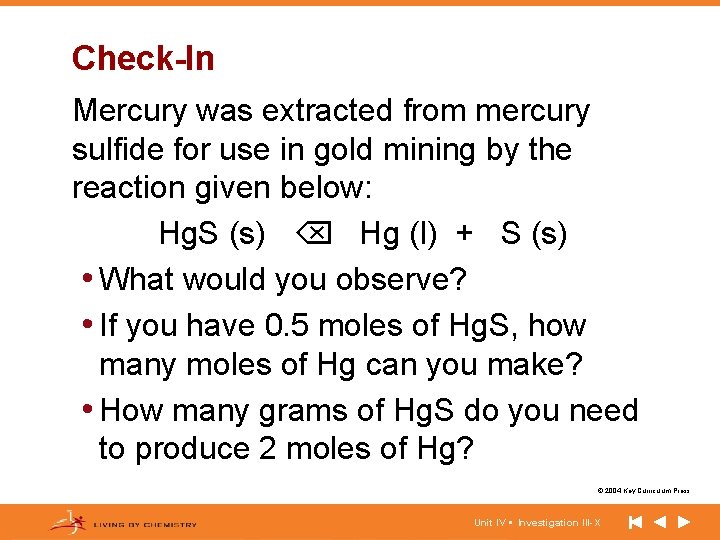
Check-In Mercury was extracted from mercury sulfide for use in gold mining by the reaction given below: Hg. S (s) Hg (l) + S (s) • What would you observe? • If you have 0. 5 moles of Hg. S, how many moles of Hg can you make? • How many grams of Hg. S do you need to produce 2 moles of Hg? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Wrap-Up • In order to make a specified mass of product, chemists must determine how many moles of that product they are trying to create. • In completing calculations for chemical reactions, chemists convert back and forth between grams and moles. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Toxins Unit – Investigation III Lesson 5: Mass Appeal

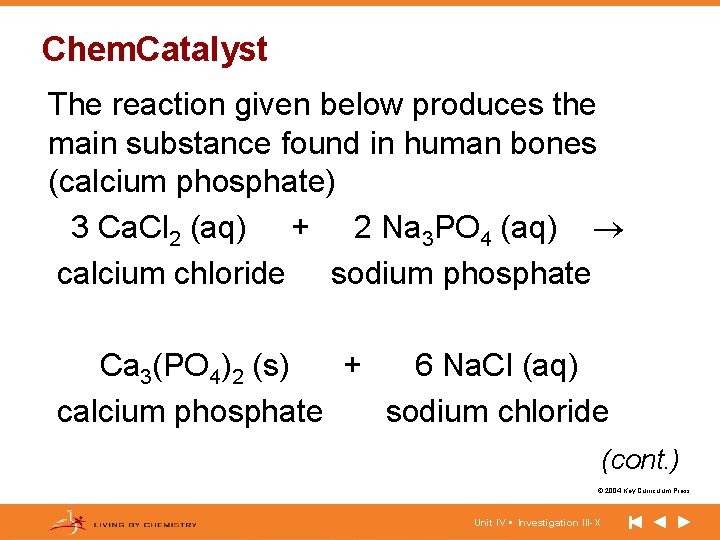
Chem. Catalyst The reaction given below produces the main substance found in human bones (calcium phosphate) 3 Ca. Cl 2 (aq) + 2 Na 3 PO 4 (aq) calcium chloride sodium phosphate Ca 3(PO 4)2 (s) + 6 Na. Cl (aq) calcium phosphate sodium chloride (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) • If you react 6 moles of calcium chloride, Ca. Cl 2, how many moles of calcium phosphate can you make? • If you react 111 grams of Ca. Cl 2, how many moles of calcium phosphate can you make? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

The Big Question • How can the mole concept be used to calculate the actual mass of products produced, or the mass of reactants needed, in a chemical reaction? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

You will be able to: Use the mole concept and balanced chemical equations to convert back and forth between masses of reactants. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

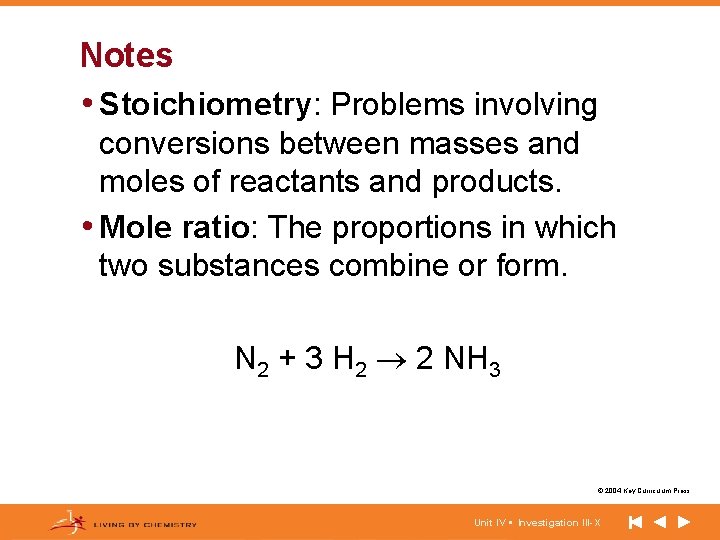
Notes • Stoichiometry: Problems involving conversions between masses and moles of reactants and products. • Mole ratio: The proportions in which two substances combine or form. N 2 + 3 H 2 2 NH 3 © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Activity Purpose: In this activity you will perform stoichiometric calculations. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) calcium iodide sodium oxalate calcium oxalate sodium iodide (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

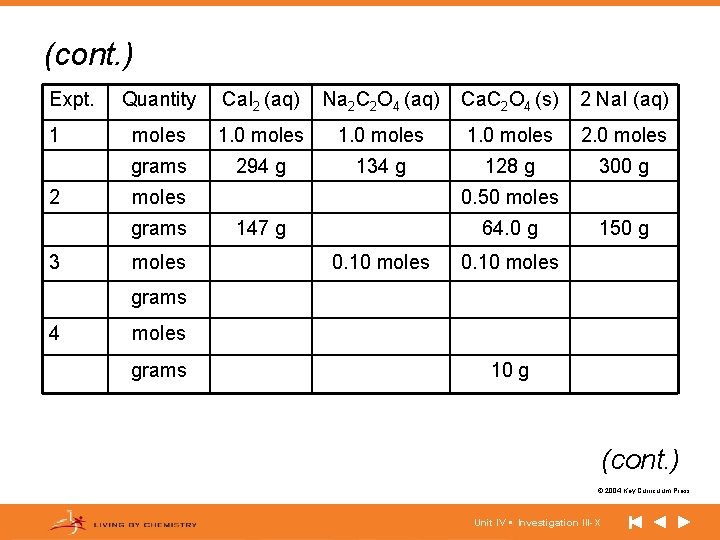
(cont. ) Expt. 1 2 Quantity Ca. I 2 (aq) Na 2 C 2 O 4 (aq) Ca. C 2 O 4 (s) 2 Na. I (aq) moles 1. 0 moles 2. 0 moles grams 294 g 134 g 128 g 300 g moles grams 3 moles 0. 50 moles 147 g 64. 0 g 0. 10 moles 150 g 0. 10 moles grams 4 moles grams 10 g (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) calcium chloride sodium phosphate calcium phosphate sodium chloride (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

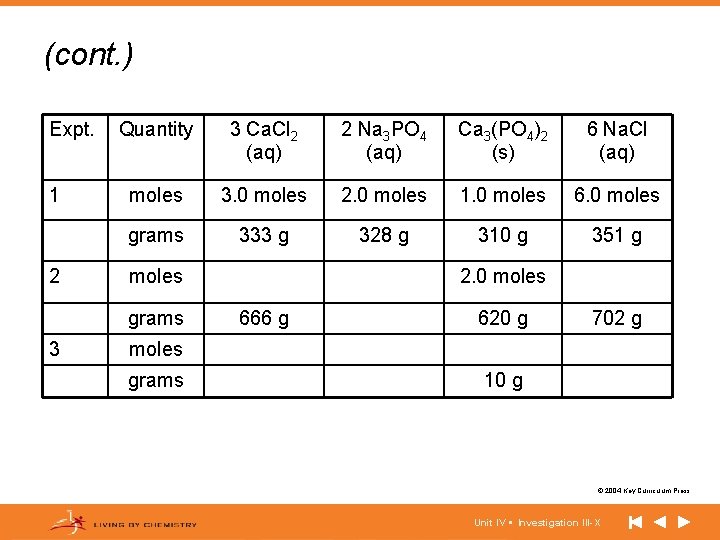
(cont. ) Expt. 1 2 Quantity 3 Ca. Cl 2 (aq) 2 Na 3 PO 4 (aq) Ca 3(PO 4)2 (s) 6 Na. Cl (aq) moles 3. 0 moles 2. 0 moles 1. 0 moles 6. 0 moles grams 333 g 328 g 310 g 351 g moles grams 3 2. 0 moles 666 g 620 g 702 g moles grams 10 g © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Making Sense • Outline the steps you took to calculate the number of grams of calcium chloride needed to make 50 grams of calcium phosphate. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) Example: How many grams of calcium phosphate can be made with 25 grams of calcium chloride? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

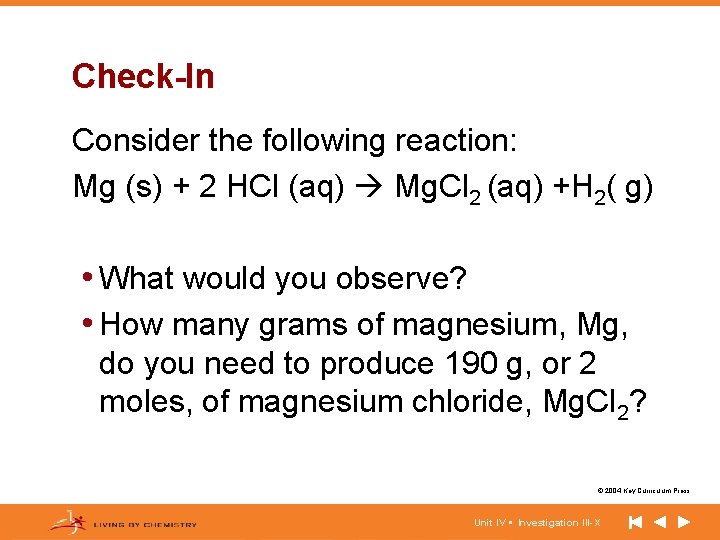
Check-In Consider the following reaction: Mg (s) + 2 HCl (aq) Mg. Cl 2 (aq) +H 2( g) • What would you observe? • How many grams of magnesium, Mg, do you need to produce 190 g, or 2 moles, of magnesium chloride, Mg. Cl 2? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Wrap-Up • Stoichiometric calculations are those involving masses of reactants and products in chemical reactions. • In order to calculate the mass of reactant needed to make a certain mass of product it is necessary to convert mass to moles and then back again to mass. • Mole ratios assist in converting back and forth between moles of reactant and product. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Toxins Unit – Investigation III Lesson 6: Get the Lead Out

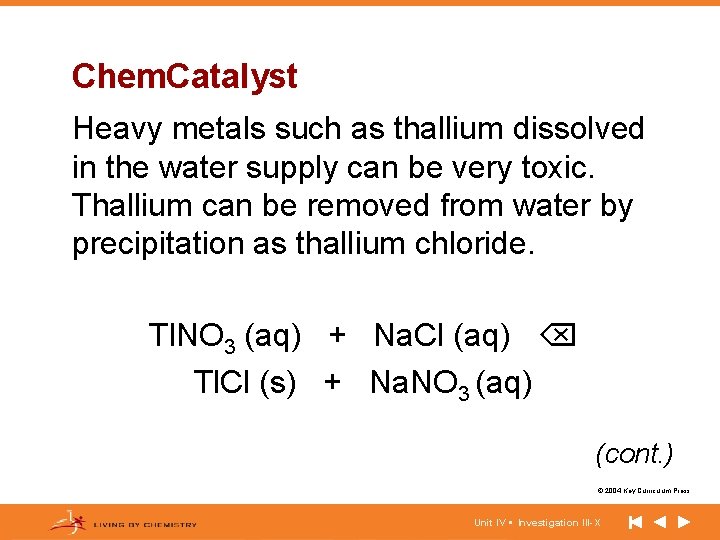
Chem. Catalyst Heavy metals such as thallium dissolved in the water supply can be very toxic. Thallium can be removed from water by precipitation as thallium chloride. Tl. NO 3 (aq) + Na. Cl (aq) Tl. Cl (s) + Na. NO 3 (aq) (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) • Which will produce more moles of Tl. Cl? A. 10 g Tl. NO 3 and 10 g Na. Cl B. 12 g Tl. NO 3 and 8 g Na. Cl C. 8 g Tl. NO 3 and 12 g Na. Cl © 2004 Key Curriculum Press. Unit IV • Investigation III-X

The Big Question • What happens in a chemical reaction when one of the reactants runs out before the other? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

You will be able to: Explain the concept of a limiting reactant and how it affects the amount of a product produced in a chemical reaction. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Notes • In a stoichiometric mixture the reactants are mixed in the mole ratios specified by the balanced equation. • A limiting reactant is a reactant that gets used up because the mixture is not stoichiometric. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Activity Purpose: In this activity, you will consider how to determine if one reactant is in excess. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Making Sense • If you have 662 g Pb(NO 3)2 dissolved in a water supply and you add 230 g Na. Cl, how can you determine if you have enough Na. Cl to remove the lead as solid Pb. Cl 2? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

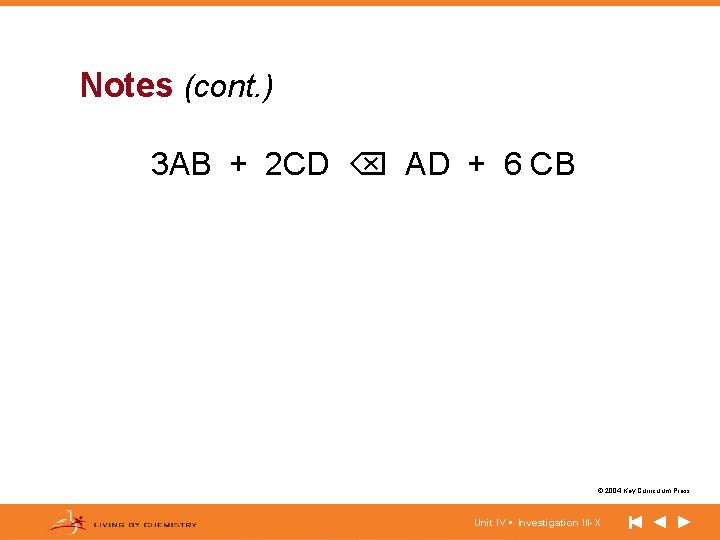
Notes (cont. ) 3 AB + 2 CD AD + 6 CB © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) Sample problem: We take vitamins and mineral supplements so that they do not become limiting reactants in our body. If you have 13. 3 g Ca. Cl 2 and 9. 84 g Na 3 PO 4 available, how many grams of Ca 3(PO 4)2 (bone) can you make? Should you take a calcium supplement? Explain your thinking. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

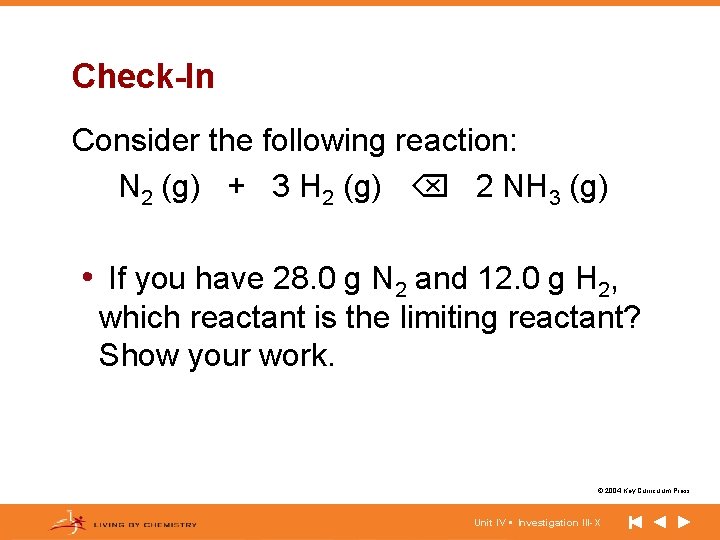
Check-In Consider the following reaction: N 2 (g) + 3 H 2 (g) 2 NH 3 (g) • If you have 28. 0 g N 2 and 12. 0 g H 2, which reactant is the limiting reactant? Show your work. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Wrap-Up • The limiting reactant is the substance that gets used up in a chemical reaction. • To determine the limiting reactant, calculate how many moles of product you will make for each mole of reactant. The one that gives fewer moles is the limiting reactant. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Toxins Unit – Investigation III Lesson 7: Grammies

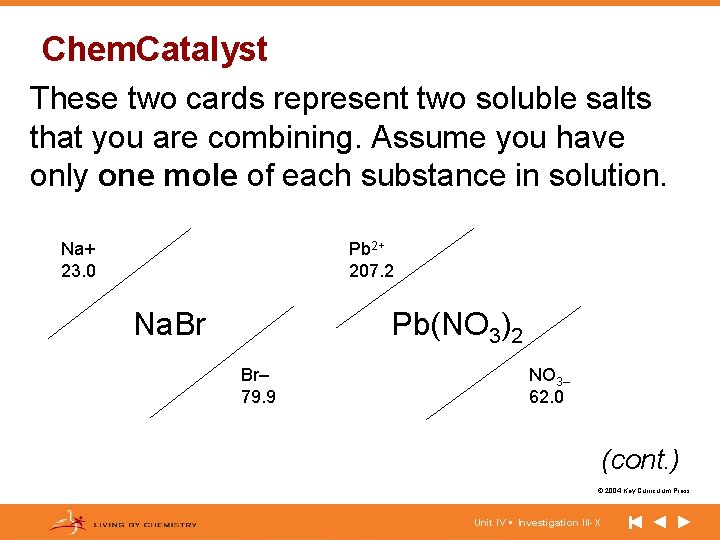
Chem. Catalyst These two cards represent two soluble salts that you are combining. Assume you have only one mole of each substance in solution. Na+ 23. 0 Pb 2+ 207. 2 Na. Br Pb(NO 3)2 Br– 79. 9 NO 3– 62. 0 (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

(cont. ) • Write a balanced equation for this precipitation reaction. • What precipitate will form? • What is the maximum number of moles of precipitate that will form when you mix 1 mole of the reactants? • How many grams of precipitate will form? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

The Big Question • How can the limiting reactant concept be used to predict quantity of product from a specific chemical reaction? © 2004 Key Curriculum Press. Unit IV • Investigation III-X

You will be able to: Use the limiting reactant concept and a balanced chemical equation to calculate the quantity of a product that can be produced by a particular chemical reaction. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Activity Purpose: This card game will allow you to practice all you’ve learned about precipitation reactions and solubility trends. (cont. ) © 2004 Key Curriculum Press. Unit IV • Investigation III-X

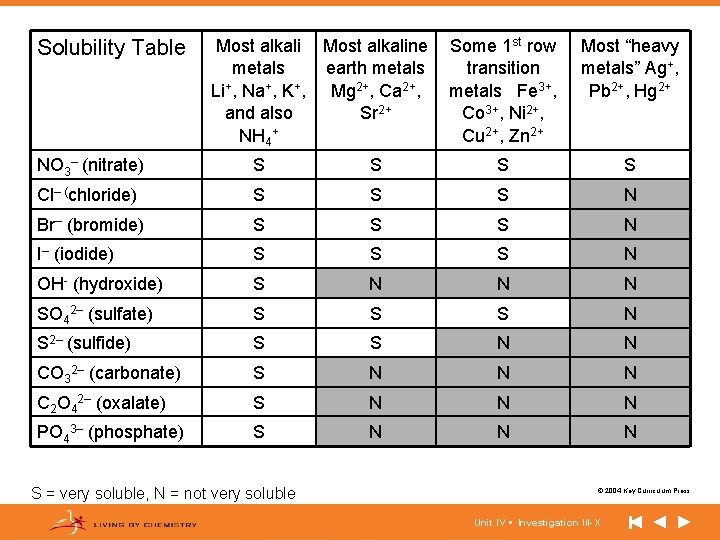
Solubility Table Most alkaline metals earth metals Li+, Na+, K+, Mg 2+, Ca 2+, and also Sr 2+ NH 4+ Some 1 st row transition metals Fe 3+, Co 3+, Ni 2+, Cu 2+, Zn 2+ Most “heavy metals” Ag+, Pb 2+, Hg 2+ NO 3– (nitrate) S S Cl– (chloride) S S S N Br– (bromide) S S S N I– (iodide) S S S N OH- (hydroxide) S N N N SO 42– (sulfate) S S S N S 2– (sulfide) S S N N CO 32– (carbonate) S N N N C 2 O 42– (oxalate) S N N N PO 43– (phosphate) S N N N S = very soluble, N = not very soluble © 2004 Key Curriculum Press. Unit IV • Investigation III-X

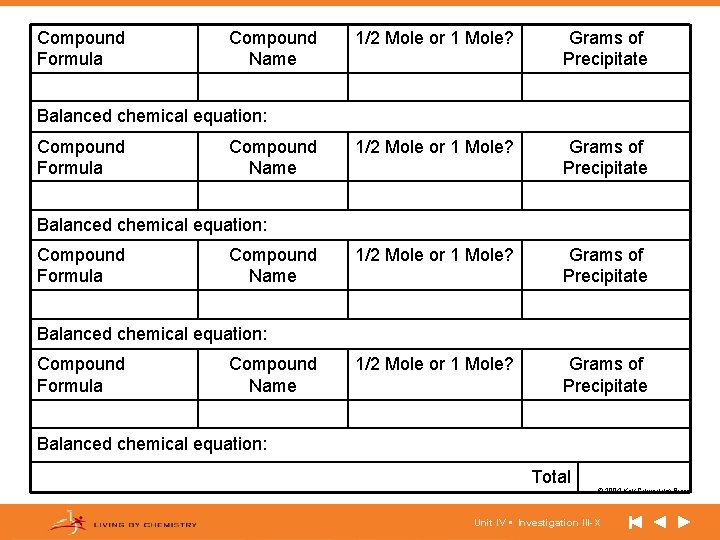
Compound Formula Compound Name 1/2 Mole or 1 Mole? Grams of Precipitate Balanced chemical equation: Compound Formula Compound Name Balanced chemical equation: Total © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Making Sense • No Making Sense question © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Check-In • No Check-In exercise. © 2004 Key Curriculum Press. Unit IV • Investigation III-X

Wrap-Up • No wrap-up points. © 2004 Key Curriculum Press. Unit IV • Investigation III-X
 Unit 4: toxins lesson 78 worksheet answers
Unit 4: toxins lesson 78 worksheet answers Toxic reactions chemical equations
Toxic reactions chemical equations Some snakes are venomous complex sentence
Some snakes are venomous complex sentence Human pathogens and toxins act
Human pathogens and toxins act 3 reasons to use supportive stance
3 reasons to use supportive stance Verbal escalation continuum cpi
Verbal escalation continuum cpi Derive henderson equation
Derive henderson equation Precipitating factors meaning
Precipitating factors meaning Difference between occlusion and mixed-crystal formation
Difference between occlusion and mixed-crystal formation Coprecipitation errors
Coprecipitation errors Primary adsorption layer
Primary adsorption layer Inclusion in gravimetric analysis
Inclusion in gravimetric analysis Rational detachment cpi
Rational detachment cpi Hamlet act iii scene iii
Hamlet act iii scene iii Criminal investigation lesson plans
Criminal investigation lesson plans How to label hyp opp adj
How to label hyp opp adj Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Extended investigation critical thinking test
Extended investigation critical thinking test Tems investigation
Tems investigation Seven s of crime scene
Seven s of crime scene Illumination in entrepreneurship
Illumination in entrepreneurship System planning and initial investigation
System planning and initial investigation Surface area investigation
Surface area investigation What do you understand by statistical investigation
What do you understand by statistical investigation How to solve special right triangles 45 45 90
How to solve special right triangles 45 45 90 Leapfrog investigation
Leapfrog investigation What is personnel security
What is personnel security Spacing of boreholes for soil investigation
Spacing of boreholes for soil investigation Moving straight ahead investigation 1
Moving straight ahead investigation 1 Vce chemistry practical investigation ideas
Vce chemistry practical investigation ideas 2106 crime scene investigation
2106 crime scene investigation Intermediate crime scene tcole
Intermediate crime scene tcole Historical scene investigation
Historical scene investigation Whitewater investigation apush
Whitewater investigation apush Forensic science foodborne outbreak investigation answers
Forensic science foodborne outbreak investigation answers Crime scene vocabulary
Crime scene vocabulary Ice lined refrigerator ppt
Ice lined refrigerator ppt Drug diversion investigation checklist
Drug diversion investigation checklist Types of statistical investigation
Types of statistical investigation Fulton county child protective services
Fulton county child protective services Spacing of boreholes for soil investigation
Spacing of boreholes for soil investigation Catching killers fire investigation
Catching killers fire investigation Objectives of investigation
Objectives of investigation 5-9 accident investigation data answers
5-9 accident investigation data answers The term encompasses all objects that can establish
The term encompasses all objects that can establish Ycci scholar award
Ycci scholar award Weather investigation
Weather investigation Tipe investigasi dalam penelitian
Tipe investigasi dalam penelitian Dfrws investigative model
Dfrws investigative model Continuous mortality investigation
Continuous mortality investigation Special triangles practice
Special triangles practice Software
Software Soil investigation methods
Soil investigation methods Investigation for reservoir planning
Investigation for reservoir planning Oos phase 2 investigation
Oos phase 2 investigation Ncit training
Ncit training Geometry riders grade 11
Geometry riders grade 11 Connected mathematics stretching and shrinking
Connected mathematics stretching and shrinking Special right triangle investigation
Special right triangle investigation Kidnap for ransom investigation procedures
Kidnap for ransom investigation procedures 2106 crime scene investigation
2106 crime scene investigation Wave interference investigation
Wave interference investigation Conclusion of investigation
Conclusion of investigation Independent investigation meaning
Independent investigation meaning Systematic investigation example
Systematic investigation example Mr philip kaloo
Mr philip kaloo Career investigation fccla
Career investigation fccla Crime scence investigator
Crime scence investigator Orensic
Orensic Phet charges and charged objects investigation
Phet charges and charged objects investigation Normochromic
Normochromic Factors of 150
Factors of 150 Lab investigation form
Lab investigation form What is system investigation
What is system investigation Career investigation lcvp
Career investigation lcvp Crime scene investigation background
Crime scene investigation background Find the variable
Find the variable Polycythemia differential diagnosis
Polycythemia differential diagnosis 8-2 practice special right triangles form g
8-2 practice special right triangles form g T.me/makeshard
T.me/makeshard Biotaphonomy
Biotaphonomy Statistical investigation process
Statistical investigation process Soil investigation
Soil investigation Scientific vs technical
Scientific vs technical Démarche d'investigation
Démarche d'investigation Biscuit investigation
Biscuit investigation Bing image search
Bing image search Oos investigation mhra
Oos investigation mhra Sti 2 d
Sti 2 d Démarche d'investigation
Démarche d'investigation Investigation procedures
Investigation procedures Indiana jane and the investigation of matter answer key
Indiana jane and the investigation of matter answer key A plan of investigation
A plan of investigation Triangle 1 11 121
Triangle 1 11 121 Lockheed martin investigation
Lockheed martin investigation What are the 5 steps in crime scene investigation
What are the 5 steps in crime scene investigation Kentucky nurse practice act
Kentucky nurse practice act