Total Synthesis of Balanol A potent Protein Kinase
Total Synthesis of (-)-Balanol A potent Protein Kinase C inhibitor and Analogues
Protein Kinase C (PKC) is a family of Ser/Thr specific kinases Play a role in cellular control • Lipid dependant • Target of phorbol esters (tumor promoters) • Sphinx was formed to exploit PKC Robotic assays with 9 expressed PKC human isoforms • Natural Products and Med. Chem groups • 10/30/2021 Philip Hughes 2
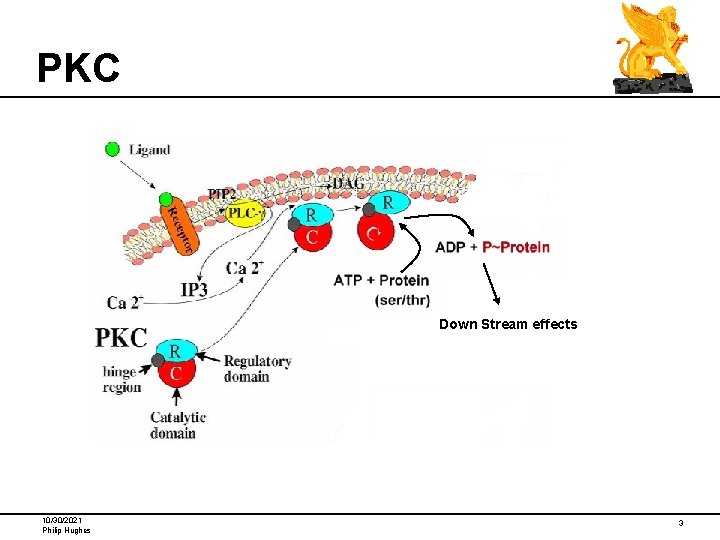
PKC Down Stream effects 10/30/2021 Philip Hughes 3
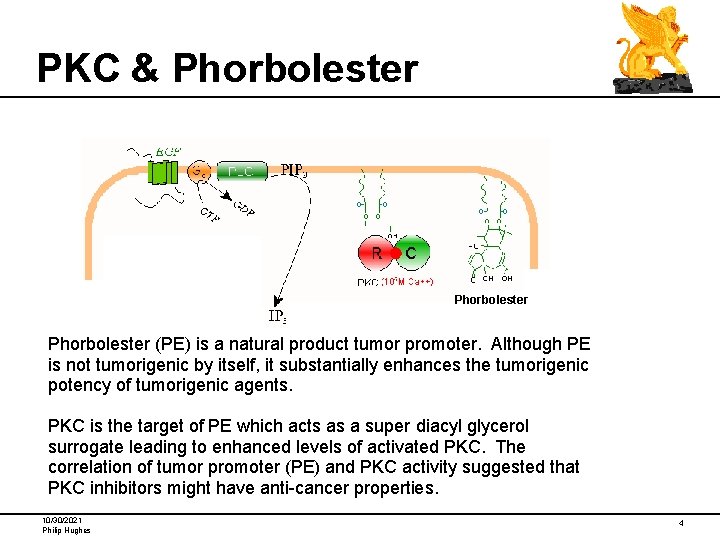
PKC & Phorbolester (PE) is a natural product tumor promoter. Although PE is not tumorigenic by itself, it substantially enhances the tumorigenic potency of tumorigenic agents. PKC is the target of PE which acts as a super diacyl glycerol surrogate leading to enhanced levels of activated PKC. The correlation of tumor promoter (PE) and PKC activity suggested that PKC inhibitors might have anti-cancer properties. 10/30/2021 Philip Hughes 4
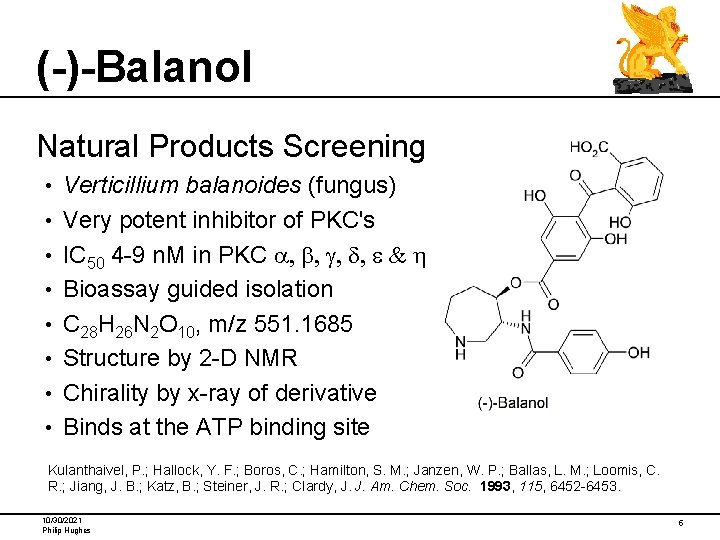
(-)-Balanol Natural Products Screening • • Verticillium balanoides (fungus) Very potent inhibitor of PKC's IC 50 4 -9 n. M in PKC a, b, g, d, e & h Bioassay guided isolation C 28 H 26 N 2 O 10, m/z 551. 1685 Structure by 2 -D NMR Chirality by x-ray of derivative Binds at the ATP binding site Kulanthaivel, P. ; Hallock, Y. F. ; Boros, C. ; Hamilton, S. M. ; Janzen, W. P. ; Ballas, L. M. ; Loomis, C. R. ; Jiang, J. B. ; Katz, B. ; Steiner, J. R. ; Clardy, J. J. Am. Chem. Soc. 1993, 115, 6452 -6453. 10/30/2021 Philip Hughes 5
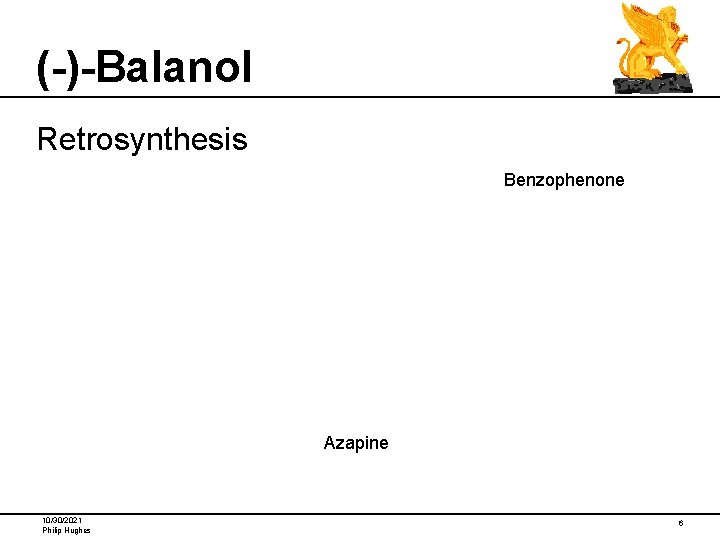
(-)-Balanol Retrosynthesis Benzophenone Azapine 10/30/2021 Philip Hughes 6
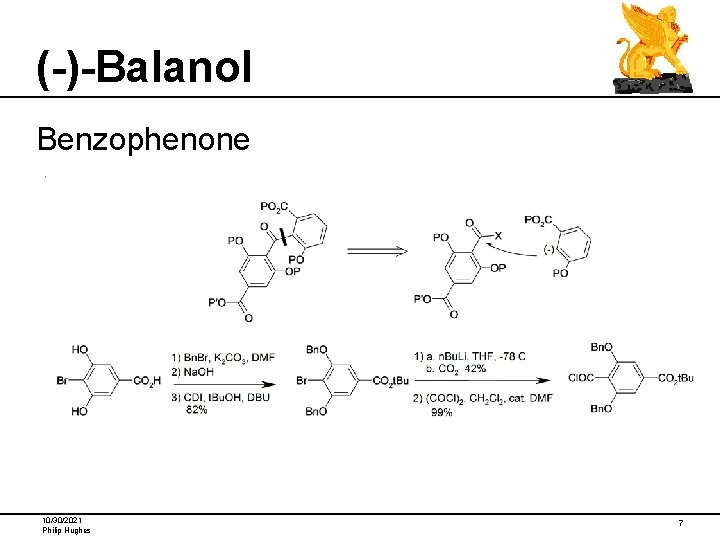
(-)-Balanol Benzophenone 10/30/2021 Philip Hughes 7
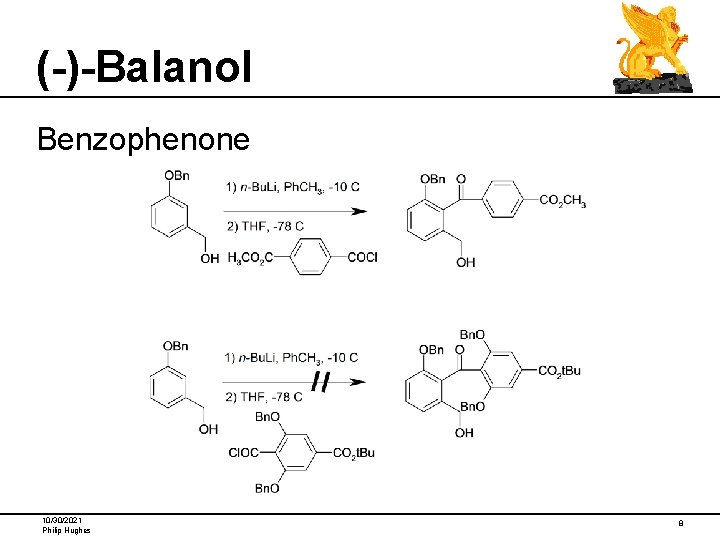
(-)-Balanol Benzophenone 10/30/2021 Philip Hughes 8

(-)-Balanol Benzophenone John Lampe, Kelly Biggers 10/30/2021 Philip Hughes 9
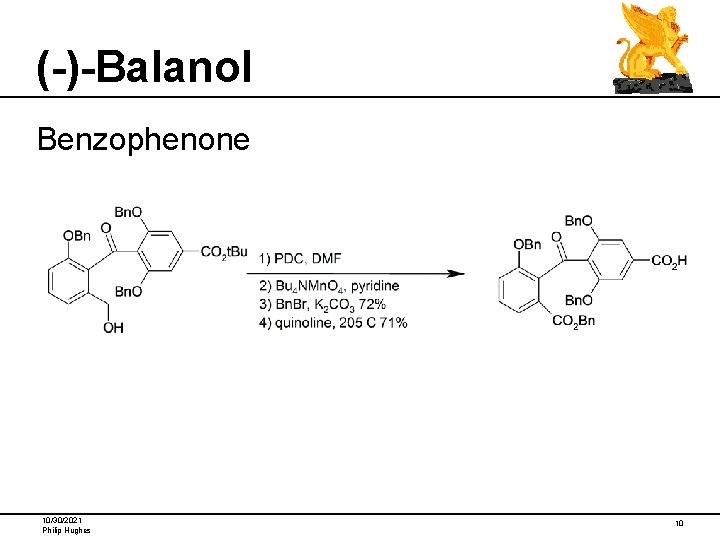
(-)-Balanol Benzophenone 10/30/2021 Philip Hughes 10
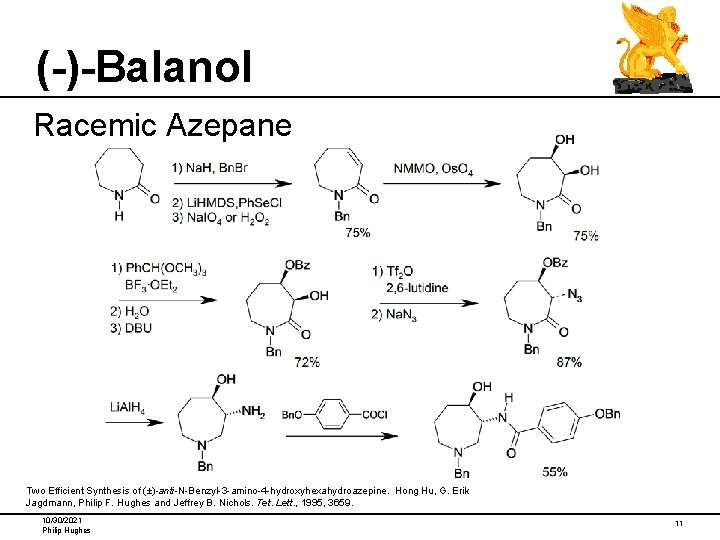
(-)-Balanol Racemic Azepane Two Efficient Synthesis of (±)-anti-N-Benzyl-3 -amino-4 -hydroxyhexahydroazepine. Hong Hu, G. Erik Jagdmann, Philip F. Hughes and Jeffrey B. Nichols. Tet. Lett. , 1995, 3659. 10/30/2021 Philip Hughes 11
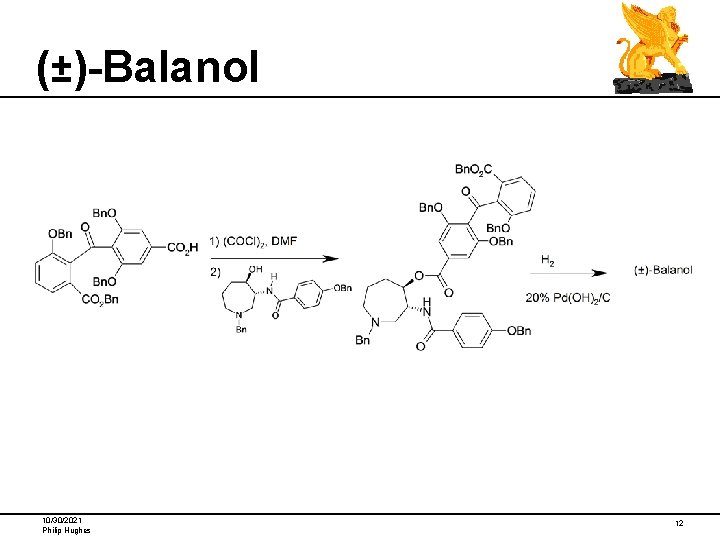
(±)-Balanol 10/30/2021 Philip Hughes 12
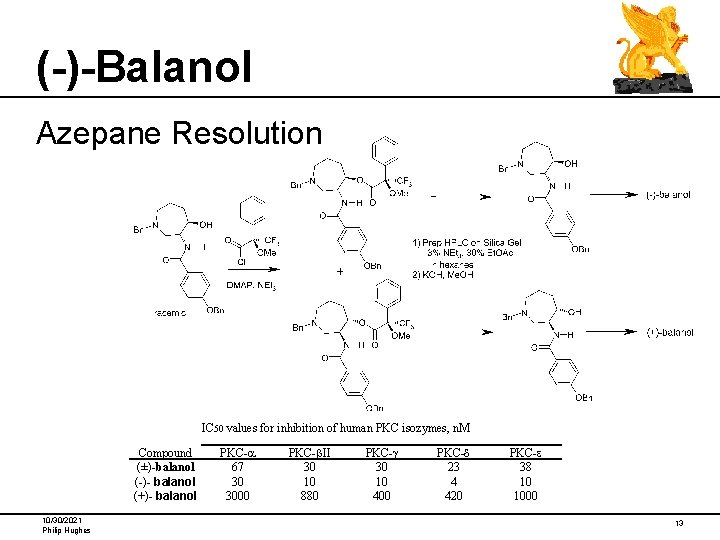
(-)-Balanol Azepane Resolution IC 50 values for inhibition of human PKC isozymes, n. M Compound (±)-balanol (-)- balanol (+)- balanol 10/30/2021 Philip Hughes PKC-a 67 30 3000 PKC-b. II 30 10 880 PKC-g 30 10 400 PKC-d 23 4 420 PKC-e 38 10 1000 13
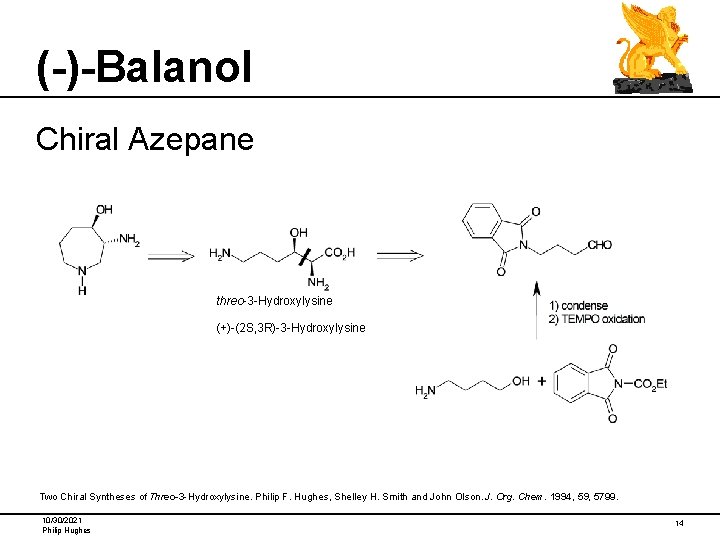
(-)-Balanol Chiral Azepane threo-3 -Hydroxylysine (+)-(2 S, 3 R)-3 -Hydroxylysine Two Chiral Syntheses of Threo-3 -Hydroxylysine. Philip F. Hughes, Shelley H. Smith and John Olson. J. Org. Chem. 1994, 59, 5799. 10/30/2021 Philip Hughes 14
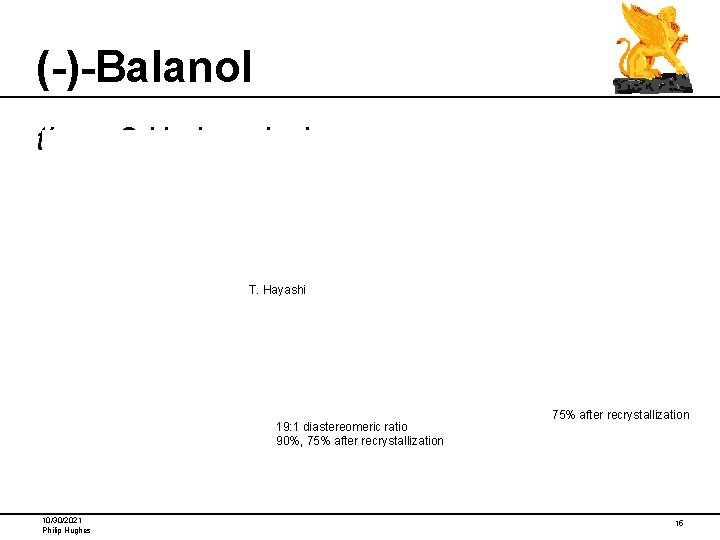
(-)-Balanol threo-3 -Hydroxylysine T. Hayashi 19: 1 diastereomeric ratio 90%, 75% after recrystallization 10/30/2021 Philip Hughes 75% after recrystallization 15
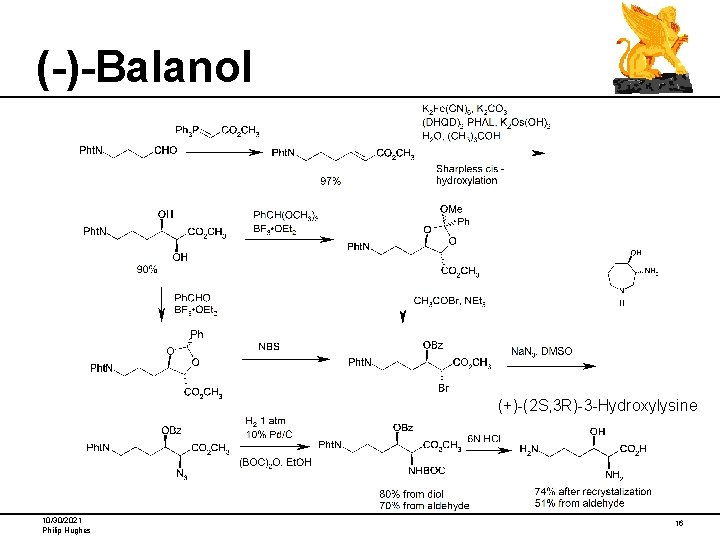
(-)-Balanol (+)-(2 S, 3 R)-3 -Hydroxylysine 10/30/2021 Philip Hughes 16
Two Chiral Syntheses of Threo-3 -Hydroxylysine. Philip F. Hughes, Shelley H. Smith and John Olson. J. Org. Chem. 1994, 59, 5799. 10/30/2021 Philip Hughes 17
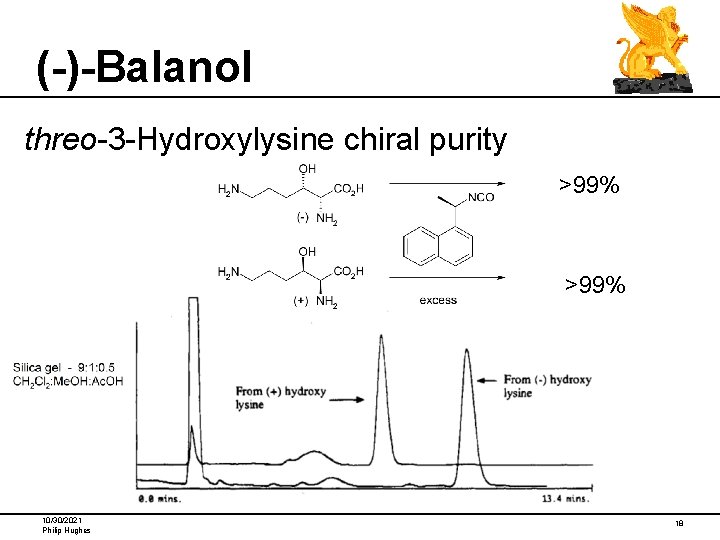
(-)-Balanol threo-3 -Hydroxylysine chiral purity >99% 10/30/2021 Philip Hughes 18
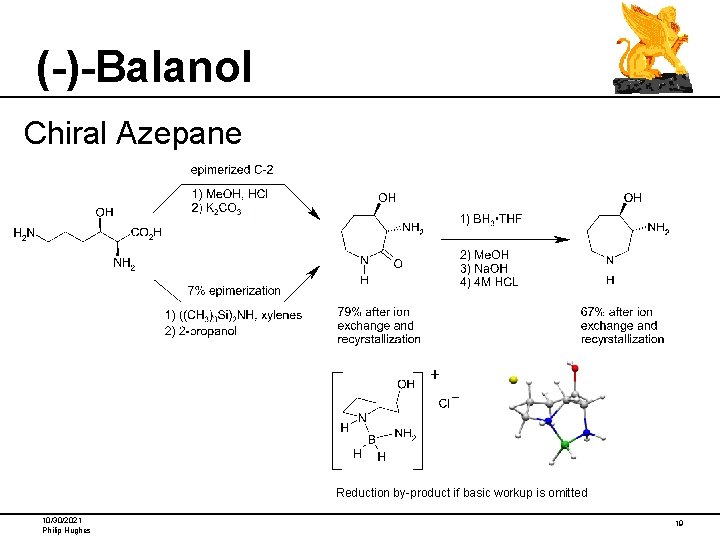
(-)-Balanol Chiral Azepane Reduction by-product if basic workup is omitted 10/30/2021 Philip Hughes 19

(-)-Balanol Total Synthesis of (-)- and (+)-Balanol. John W. Lampe, Philip F. Hughes, Christopher K. Biggers, Shelley H. Smith and Hong Hu. J. Org. Chem. 1996, 61, 4572. 10/30/2021 Philip Hughes 20
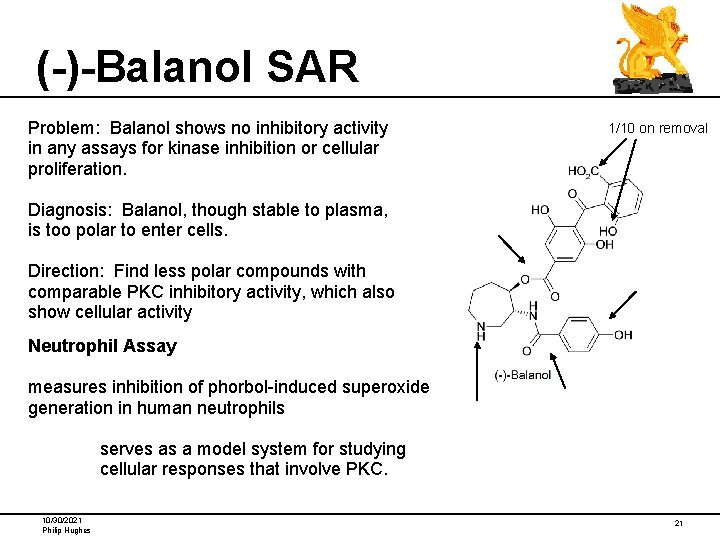
(-)-Balanol SAR Problem: Balanol shows no inhibitory activity in any assays for kinase inhibition or cellular proliferation. 1/10 on removal Diagnosis: Balanol, though stable to plasma, is too polar to enter cells. Direction: Find less polar compounds with comparable PKC inhibitory activity, which also show cellular activity Neutrophil Assay measures inhibition of phorbol-induced superoxide generation in human neutrophils serves as a model system for studying cellular responses that involve PKC. 10/30/2021 Philip Hughes 21
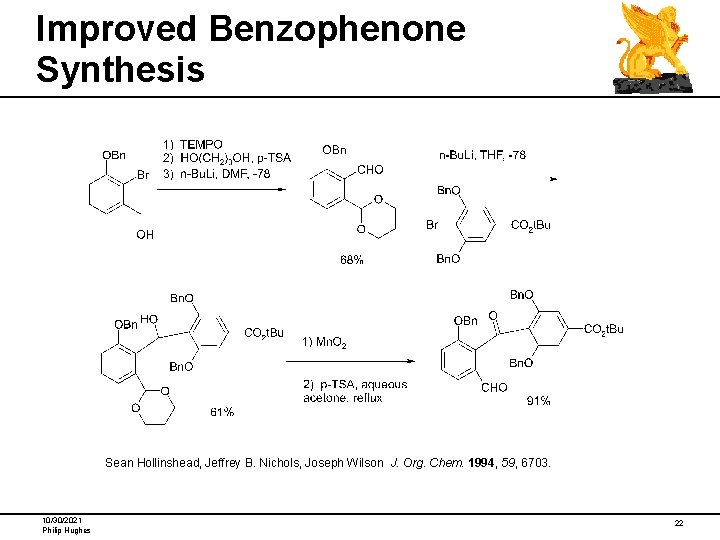
Improved Benzophenone Synthesis Sean Hollinshead, Jeffrey B. Nichols, Joseph Wilson J. Org. Chem. 1994, 59, 6703. 10/30/2021 Philip Hughes 22
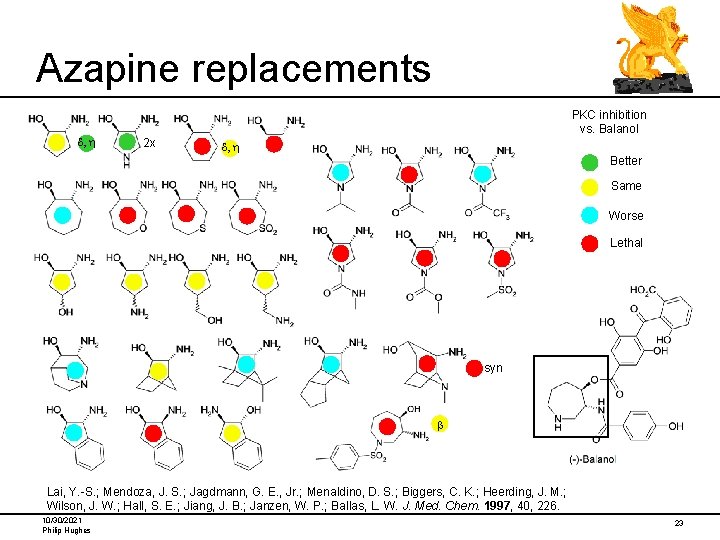
Azapine replacements d, h PKC inhibition vs. Balanol 2 x d, h Better Same Worse Lethal syn b Lai, Y. -S. ; Mendoza, J. S. ; Jagdmann, G. E. , Jr. ; Menaldino, D. S. ; Biggers, C. K. ; Heerding, J. M. ; Wilson, J. W. ; Hall, S. E. ; Jiang, J. B. ; Janzen, W. P. ; Ballas, L. W. J. Med. Chem. 1997, 40, 226. 10/30/2021 Philip Hughes 23
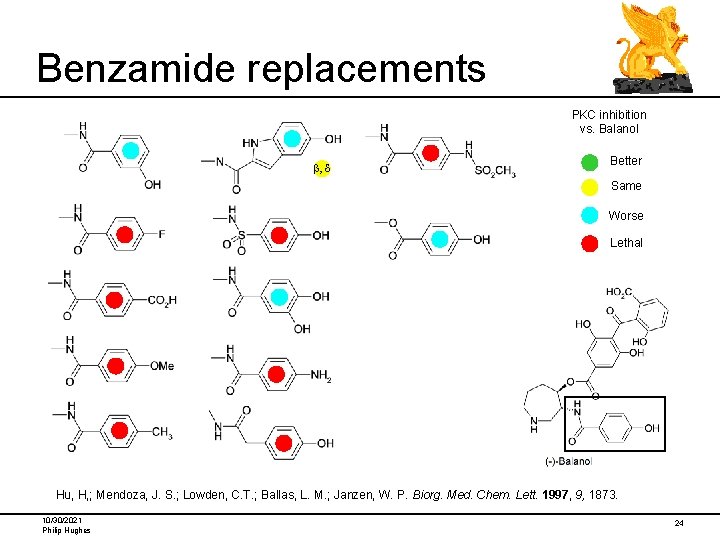
Benzamide replacements PKC inhibition vs. Balanol b, d Better Same Worse Lethal Hu, H, ; Mendoza, J. S. ; Lowden, C. T. ; Ballas, L. M. ; Janzen, W. P. Biorg. Med. Chem. Lett. 1997, 9, 1873. 10/30/2021 Philip Hughes 24
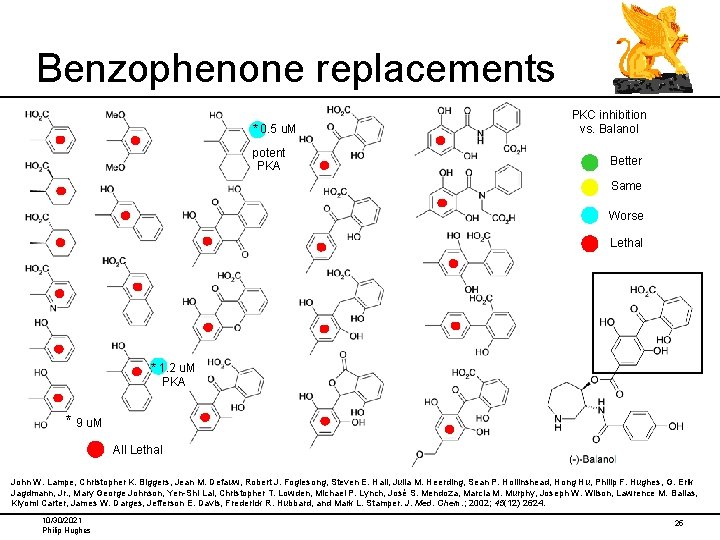
Benzophenone replacements * 0. 5 u. M potent PKA PKC inhibition vs. Balanol Better Same Worse Lethal * 1. 2 u. M PKA * 9 u. M All Lethal John W. Lampe, Christopher K. Biggers, Jean M. Defauw, Robert J. Foglesong, Steven E. Hall, Julia M. Heerding, Sean P. Hollinshead, Hong Hu, Philip F. Hughes, G. Erik Jagdmann, Jr. , Mary George Johnson, Yen-Shi Lai, Christopher T. Lowden, Michael P. Lynch, José S. Mendoza, Marcia M. Murphy, Joseph W. Wilson, Lawrence M. Ballas, Kiyomi Carter, James W. Darges, Jefferson E. Davis, Frederick R. Hubbard, and Mark L. Stamper. J. Med. Chem. ; 2002; 45(12) 2624. 10/30/2021 Philip Hughes 25
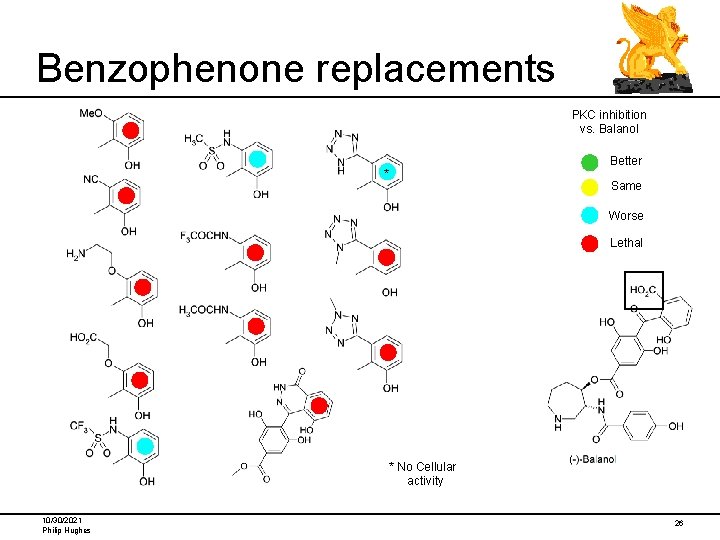
Benzophenone replacements PKC inhibition vs. Balanol Better * Same Worse Lethal * No Cellular activity 10/30/2021 Philip Hughes 26
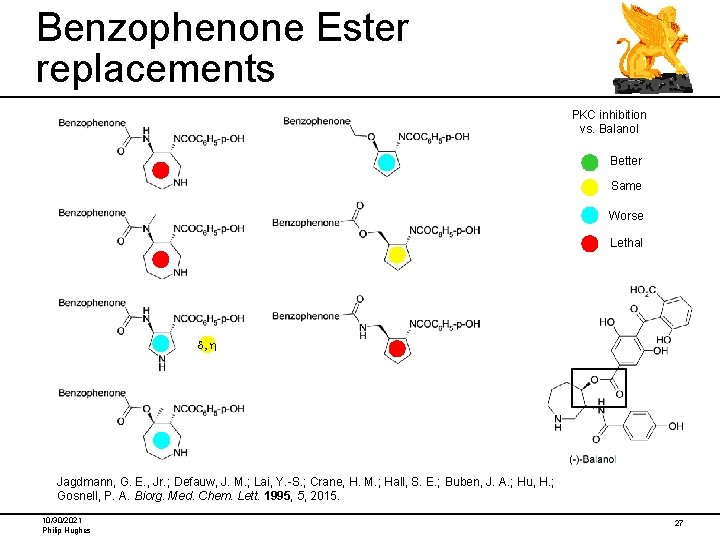
Benzophenone Ester replacements PKC inhibition vs. Balanol Better Same Worse Lethal d, h Jagdmann, G. E. , Jr. ; Defauw, J. M. ; Lai, Y. -S. ; Crane, H. M. ; Hall, S. E. ; Buben, J. A. ; Hu, H. ; Gosnell, P. A. Biorg. Med. Chem. Lett. 1995, 5, 2015. 10/30/2021 Philip Hughes 27
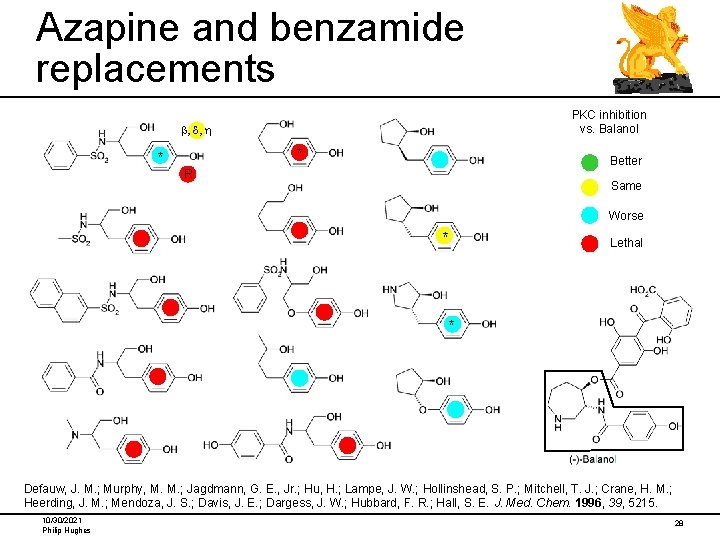
Azapine and benzamide replacements PKC inhibition vs. Balanol b, d, h * * Better R Same Worse * Lethal * Defauw, J. M. ; Murphy, M. M. ; Jagdmann, G. E. , Jr. ; Hu, H. ; Lampe, J. W. ; Hollinshead, S. P. ; Mitchell, T. J. ; Crane, H. M. ; Heerding, J. M. ; Mendoza, J. S. ; Davis, J. E. ; Dargess, J. W. ; Hubbard, F. R. ; Hall, S. E. J. Med. Chem. 1996, 39, 5215. 10/30/2021 Philip Hughes 28
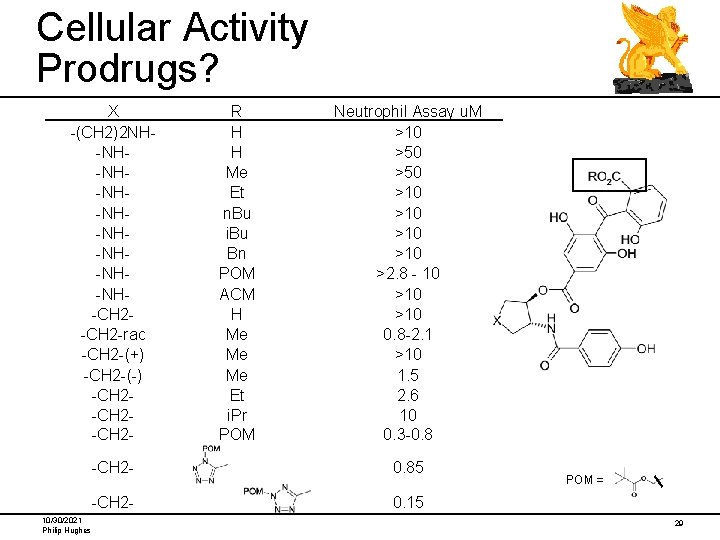
Cellular Activity Prodrugs? X -(CH 2)2 NH-NH-NH-NH-NH-CH 2 -rac -CH 2 -(+) -CH 2 -(-) -CH 2 -CH 2 - 10/30/2021 Philip Hughes R H H Me Et n. Bu i. Bu Bn POM ACM H Me Me Me Et i. Pr POM Neutrophil Assay u. M >10 >50 >10 >10 >2. 8 - 10 >10 0. 8 -2. 1 >10 1. 5 2. 6 10 0. 3 -0. 8 -CH 2 - 0. 85 -CH 2 - 0. 15 POM = 29
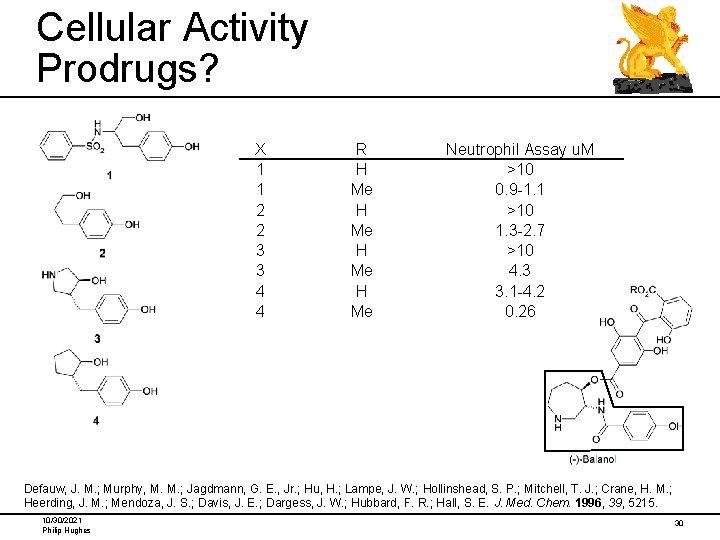
Cellular Activity Prodrugs? X 1 1 2 2 3 3 4 4 R H Me Neutrophil Assay u. M >10 0. 9 -1. 1 >10 1. 3 -2. 7 >10 4. 3 3. 1 -4. 2 0. 26 Defauw, J. M. ; Murphy, M. M. ; Jagdmann, G. E. , Jr. ; Hu, H. ; Lampe, J. W. ; Hollinshead, S. P. ; Mitchell, T. J. ; Crane, H. M. ; Heerding, J. M. ; Mendoza, J. S. ; Davis, J. E. ; Dargess, J. W. ; Hubbard, F. R. ; Hall, S. E. J. Med. Chem. 1996, 39, 5215. 10/30/2021 Philip Hughes 30
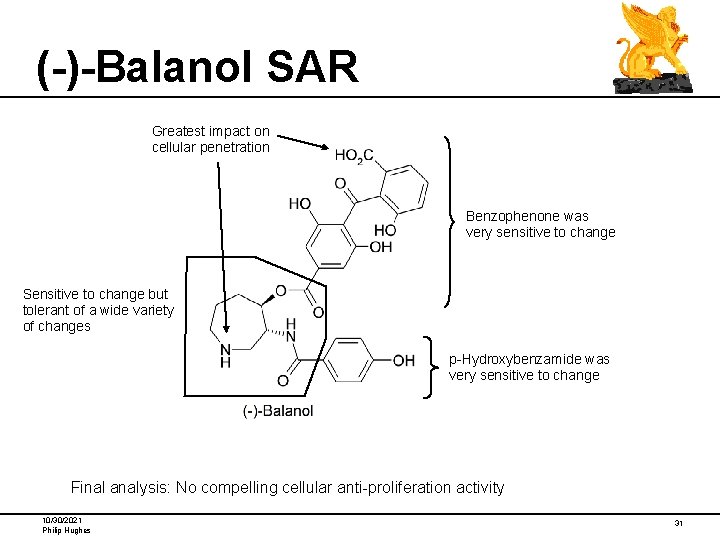
(-)-Balanol SAR Greatest impact on cellular penetration Benzophenone was very sensitive to change Sensitive to change but tolerant of a wide variety of changes p-Hydroxybenzamide was very sensitive to change Final analysis: No compelling cellular anti-proliferation activity 10/30/2021 Philip Hughes 31
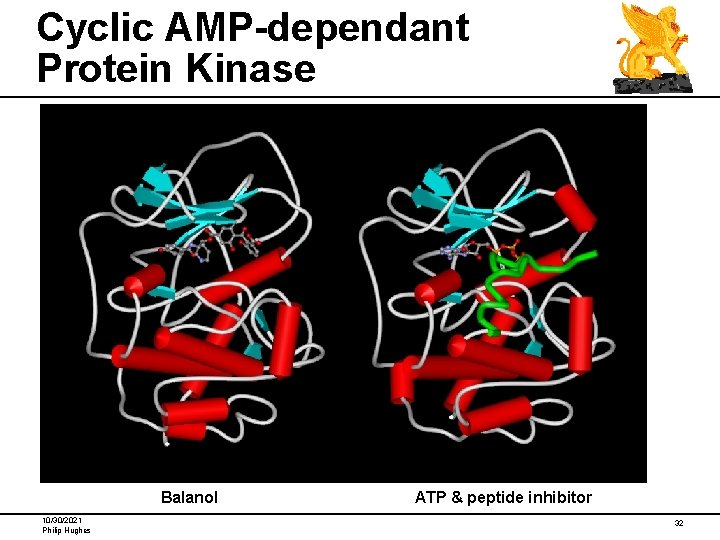
Cyclic AMP-dependant Protein Kinase Balanol 10/30/2021 Philip Hughes ATP & peptide inhibitor 32
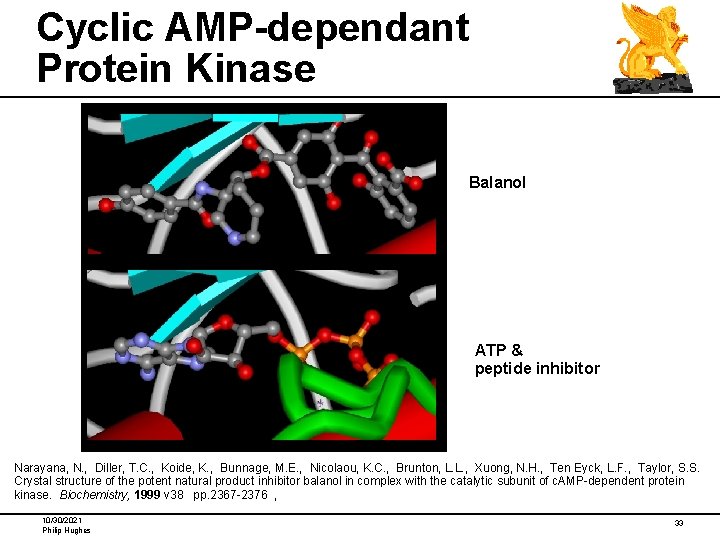
Cyclic AMP-dependant Protein Kinase Balanol ATP & peptide inhibitor Narayana, N. , Diller, T. C. , Koide, K. , Bunnage, M. E. , Nicolaou, K. C. , Brunton, L. L. , Xuong, N. H. , Ten Eyck, L. F. , Taylor, S. S. Crystal structure of the potent natural product inhibitor balanol in complex with the catalytic subunit of c. AMP-dependent protein kinase. Biochemistry, 1999 v 38 pp. 2367 -2376 , 10/30/2021 Philip Hughes 33
Acknowledgments Analytical group Tom Mitchell Carolyn Schwarz 10/30/2021 Philip Hughes 34
Parallel Synthesis As Practiced at Lilly RTP 1. Technologies • 2. 10/30/2021 Philip Hughes Reactors Examples 35
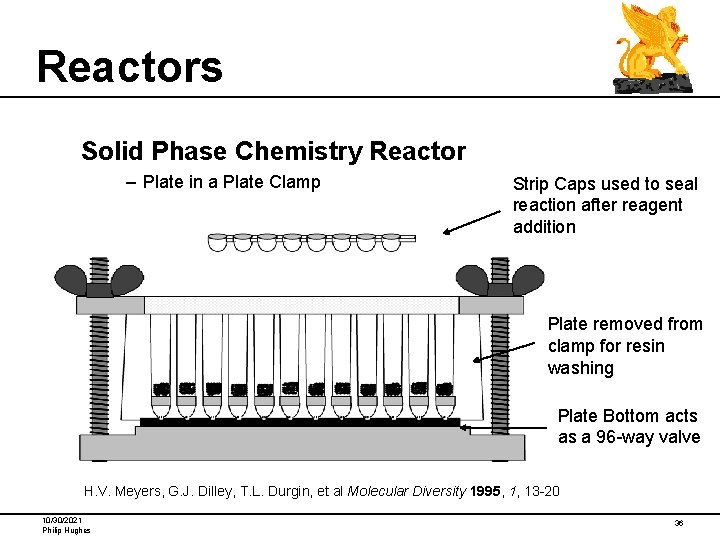
Reactors Solid Phase Chemistry Reactor – Plate in a Plate Clamp Strip Caps used to seal reaction after reagent addition Plate removed from clamp for resin washing Plate Bottom acts as a 96 -way valve H. V. Meyers, G. J. Dilley, T. L. Durgin, et al Molecular Diversity 1995, 1, 13 -20 10/30/2021 Philip Hughes 36
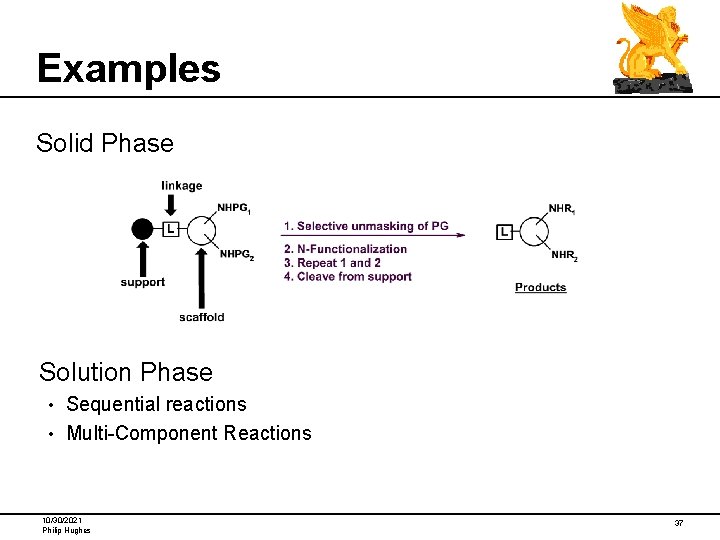
Examples Solid Phase Solution Phase Sequential reactions • Multi-Component Reactions • 10/30/2021 Philip Hughes 37
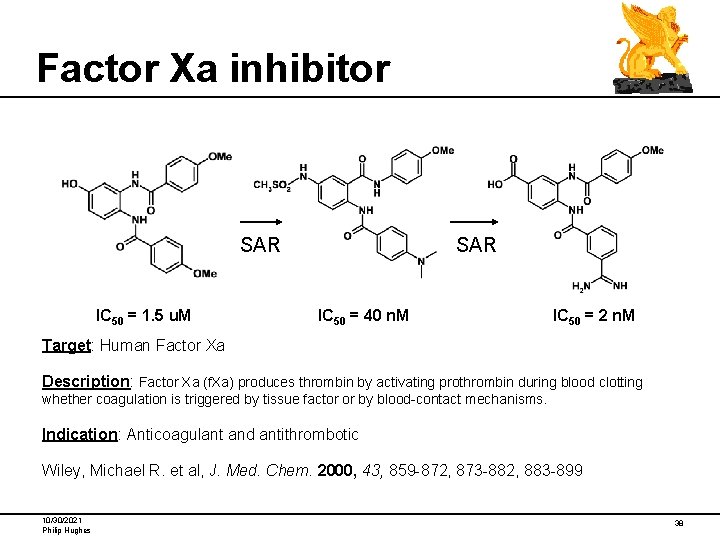
Factor Xa inhibitor SAR IC 50 = 1. 5 u. M SAR IC 50 = 40 n. M IC 50 = 2 n. M Target: Human Factor Xa Description: Factor Xa (f. Xa) produces thrombin by activating prothrombin during blood clotting whether coagulation is triggered by tissue factor or by blood-contact mechanisms. Indication: Anticoagulant and antithrombotic Wiley, Michael R. et al, J. Med. Chem. 2000, 43, 859 -872, 873 -882, 883 -899 10/30/2021 Philip Hughes 38
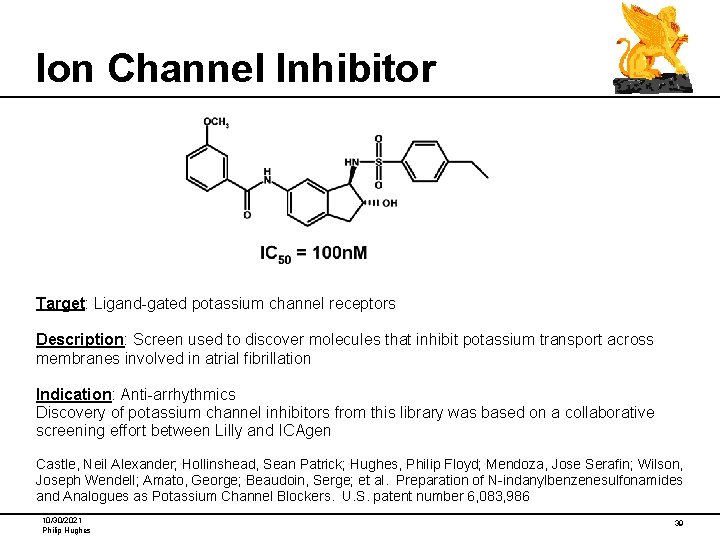
Ion Channel Inhibitor Target: Ligand-gated potassium channel receptors Description: Screen used to discover molecules that inhibit potassium transport across membranes involved in atrial fibrillation Indication: Anti-arrhythmics Discovery of potassium channel inhibitors from this library was based on a collaborative screening effort between Lilly and ICAgen Castle, Neil Alexander; Hollinshead, Sean Patrick; Hughes, Philip Floyd; Mendoza, Jose Serafin; Wilson, Joseph Wendell; Amato, George; Beaudoin, Serge; et al. Preparation of N-indanylbenzenesulfonamides and Analogues as Potassium Channel Blockers. U. S. patent number 6, 083, 986 10/30/2021 Philip Hughes 39
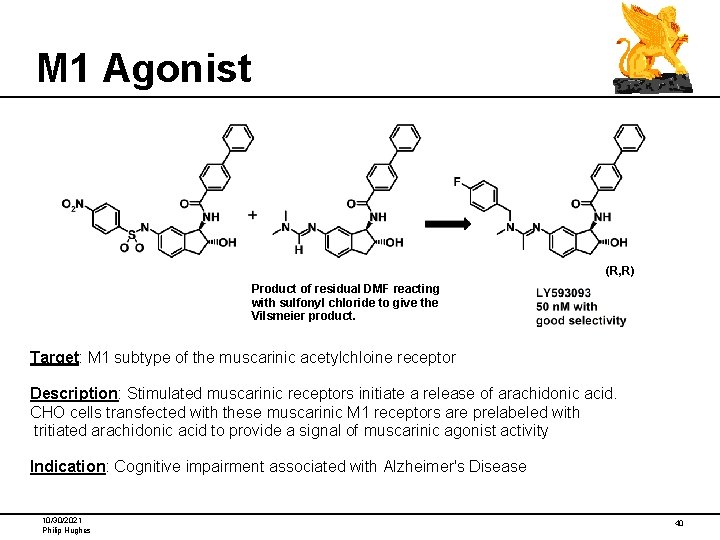
M 1 Agonist (R, R) Product of residual DMF reacting with sulfonyl chloride to give the Vilsmeier product. Target: M 1 subtype of the muscarinic acetylchloine receptor Description: Stimulated muscarinic receptors initiate a release of arachidonic acid. CHO cells transfected with these muscarinic M 1 receptors are prelabeled with tritiated arachidonic acid to provide a signal of muscarinic agonist activity Indication: Cognitive impairment associated with Alzheimer's Disease 10/30/2021 Philip Hughes 40
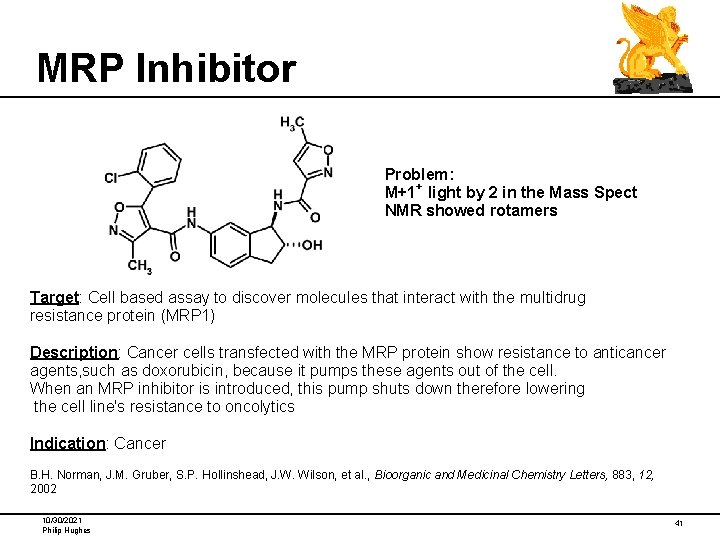
MRP Inhibitor Problem: M+1+ light by 2 in the Mass Spect NMR showed rotamers Target: Cell based assay to discover molecules that interact with the multidrug resistance protein (MRP 1) Description: Cancer cells transfected with the MRP protein show resistance to anticancer agents, such as doxorubicin, because it pumps these agents out of the cell. When an MRP inhibitor is introduced, this pump shuts down therefore lowering the cell line's resistance to oncolytics Indication: Cancer B. H. Norman, J. M. Gruber, S. P. Hollinshead, J. W. Wilson, et al. , Bioorganic and Medicinal Chemistry Letters, 883, 12, 2002 10/30/2021 Philip Hughes 41
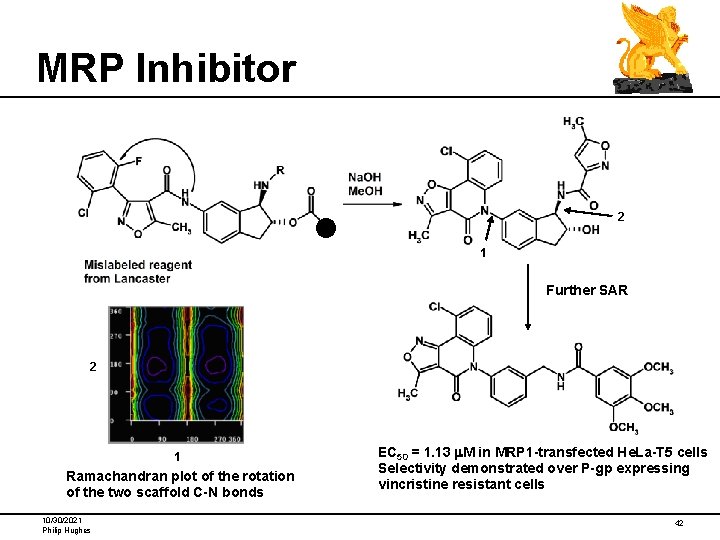
MRP Inhibitor 2 1 Further SAR 2 1 Ramachandran plot of the rotation of the two scaffold C-N bonds 10/30/2021 Philip Hughes EC 50 = 1. 13 m. M in MRP 1 -transfected He. La-T 5 cells Selectivity demonstrated over P-gp expressing vincristine resistant cells 42

Reactors Solution Phase Chemistry Reactors Microtitier Format • Various Sizes – Disposable Glassware • Reactor Vessels held at Top • Add functionality (complexity) as needed • 10/30/2021 Philip Hughes 43
Solution Library 3072 Compounds Single isomer > 95% 10/30/2021 Philip Hughes 44
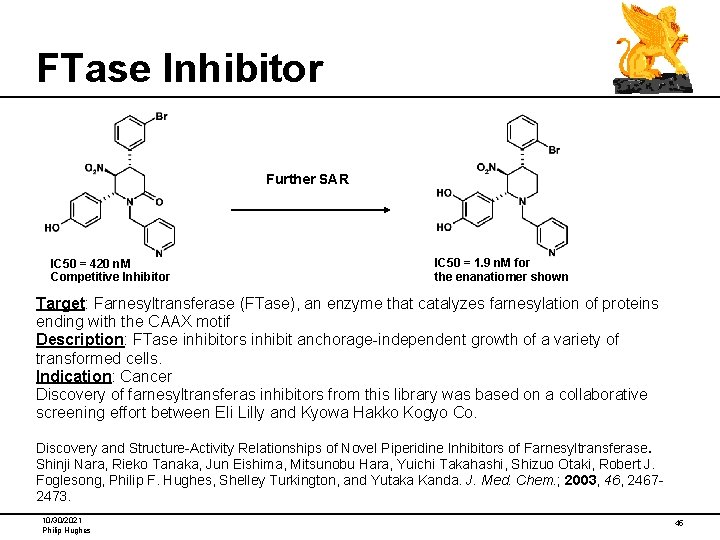
FTase Inhibitor Further SAR IC 50 = 420 n. M Competitive Inhibitor IC 50 = 1. 9 n. M for the enanatiomer shown Target: Farnesyltransferase (FTase), an enzyme that catalyzes farnesylation of proteins ending with the CAAX motif Description: FTase inhibitors inhibit anchorage-independent growth of a variety of transformed cells. Indication: Cancer Discovery of farnesyltransferas inhibitors from this library was based on a collaborative screening effort between Eli Lilly and Kyowa Hakko Kogyo Co. Discovery and Structure-Activity Relationships of Novel Piperidine Inhibitors of Farnesyltransferase. Shinji Nara, Rieko Tanaka, Jun Eishima, Mitsunobu Hara, Yuichi Takahashi, Shizuo Otaki, Robert J. Foglesong, Philip F. Hughes, Shelley Turkington, and Yutaka Kanda. J. Med. Chem. ; 2003, 46, 24672473. 10/30/2021 Philip Hughes 45
- Slides: 45