TOTAL QUALITY MANAGEMENT OVERVIEW QUALITY ASSURANCE PERFORMANCE IMPROVEMENT




































- Slides: 60

TOTAL QUALITY MANAGEMENT OVERVIEW QUALITY ASSURANCE / PERFORMANCE IMPROVEMENT (QAPI) Carlos F. Perez, MSN, RN-BC, SRVP, Total Quality Management

DEFINITION OF HEALTH CARE QUALITY “The degree to which health care services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge. ” - THE INSTITUTE OF MEDICINE (IOM) QUALITY IS A FACTOR FOR VALUE-BASED OUTCOMES CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES

QA FRIENDLY REMINDER Report quality occurrences that fail to meet expectations. Care. Kinesis may then continue to enhance systems and processes to improve customer satisfaction and safeguard participant safety.

FEEDBACK SUBMISSION

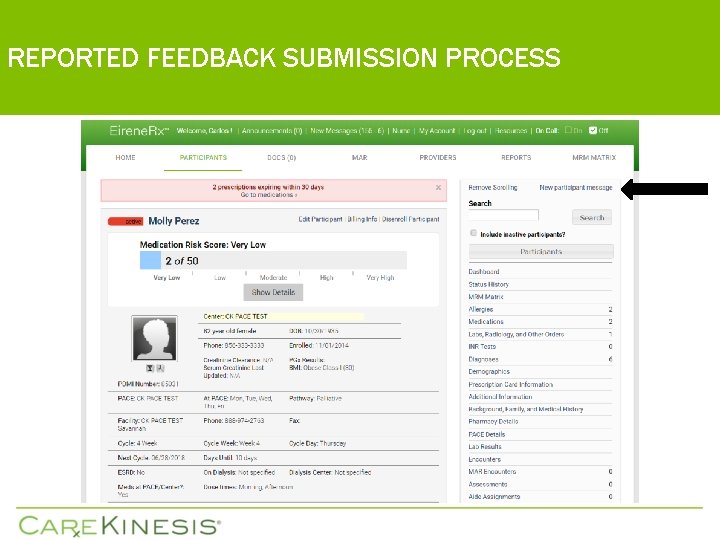
REPORTED FEEDBACK SUBMISSION PROCESS

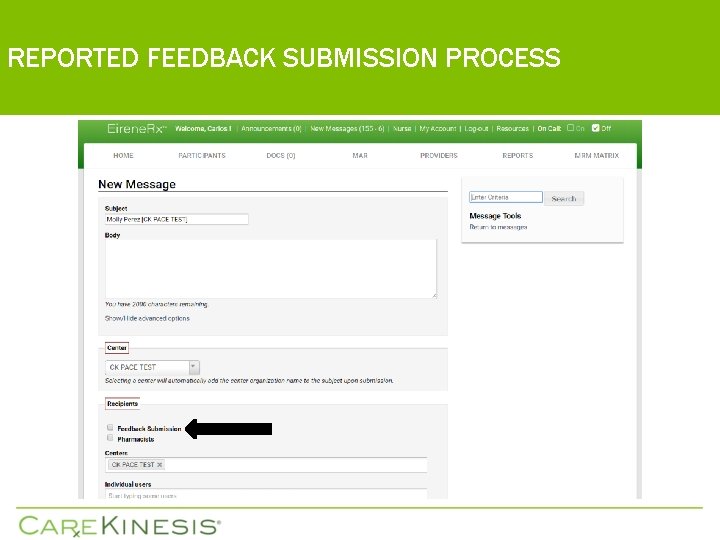
REPORTED FEEDBACK SUBMISSION PROCESS

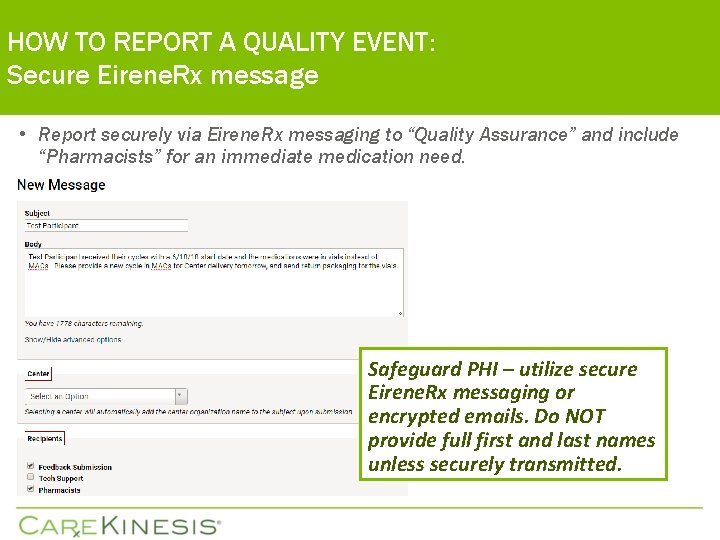
HOW TO REPORT A QUALITY EVENT: Secure Eirene. Rx message • Report securely via Eirene. Rx messaging to “Quality Assurance” and include “Pharmacists” for an immediate medication need. Safeguard PHI – utilize secure Eirene. Rx messaging or encrypted emails. Do NOT provide full first and last names unless securely transmitted.

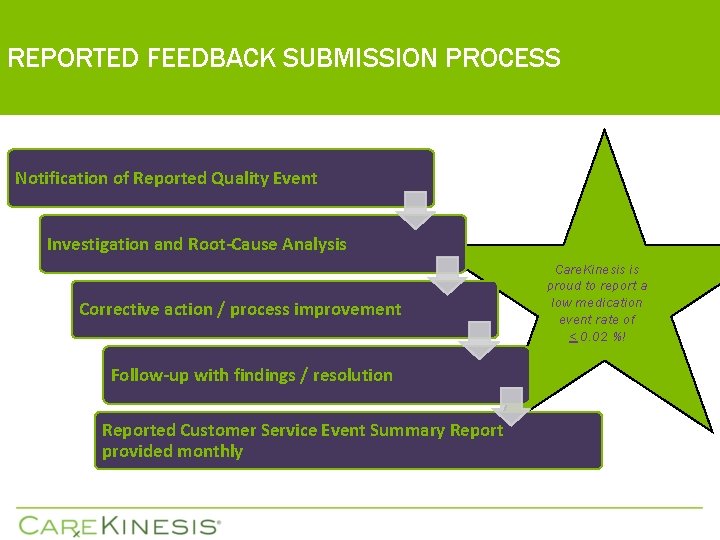
REPORTED FEEDBACK SUBMISSION PROCESS Notification of Reported Quality Event Investigation and Root-Cause Analysis Corrective action / process improvement Follow-up with findings / resolution Reported Customer Service Event Summary Report provided monthly Care. Kinesis is proud to report a low medication event rate of < 0. 02 %!

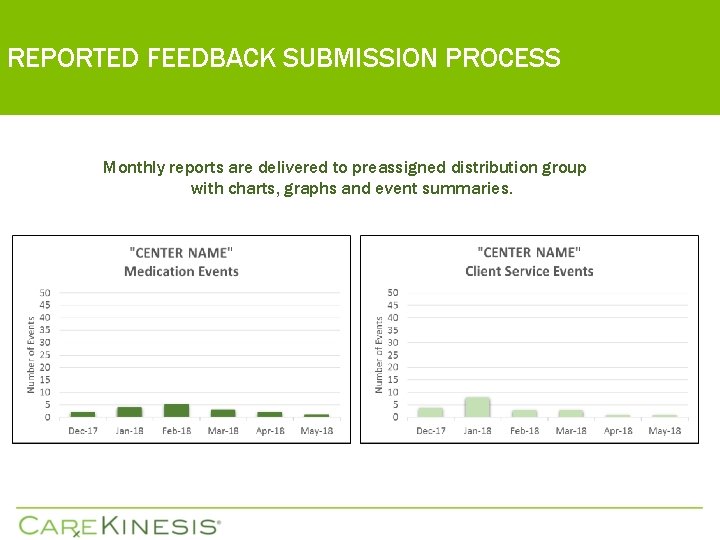
REPORTED FEEDBACK SUBMISSION PROCESS Monthly reports are delivered to preassigned distribution group with charts, graphs and event summaries.

REPORTED FEEDBACK SUBMISSION PROCESS EVENT REPORTED DATE EVENT TYPE > NATURE > SUBNATURE 5/1/2018 Medication > Medication(s) Not Needed > Cycle Fill MEDICATION EVENT DESCRIPTION A participant received lactulose with their 5/1/18 medication delivery; however, the medication was not requested for dispense. EVENT CLOSURE COMMENTS Event Resolution: The participant’s profile had two prescriptions for lactulose: one “Next Business Day" script, and one “Next Cycle Fill" script. Unfortunately, the prescription marked for "Next Cycle Fill" was added to the next day basket, and the unintended prescription was used for dispensing. That same day, the "Next Business Day" prescription was canceled by the Center. Credit for the medication was provided, and the Center was instructed to destroy the lactulose as per its policy and procedure.

OUTCOMES

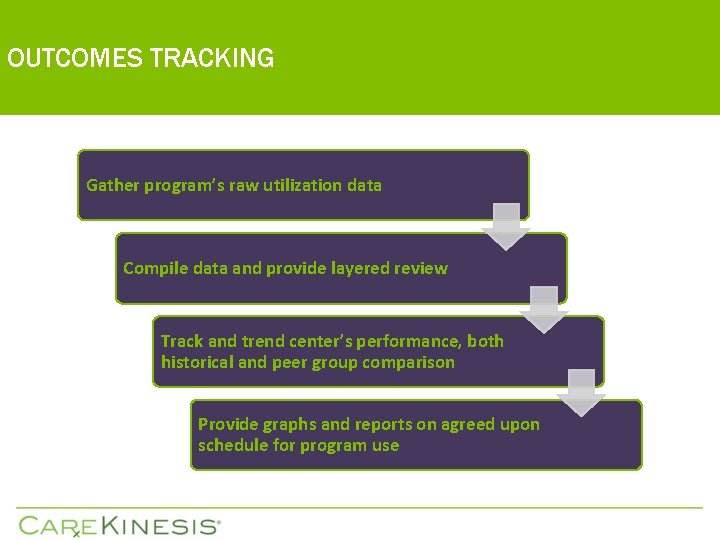
OUTCOMES TRACKING Gather program’s raw utilization data Compile data and provide layered review Track and trend center’s performance, both historical and peer group comparison Provide graphs and reports on agreed upon schedule for program use

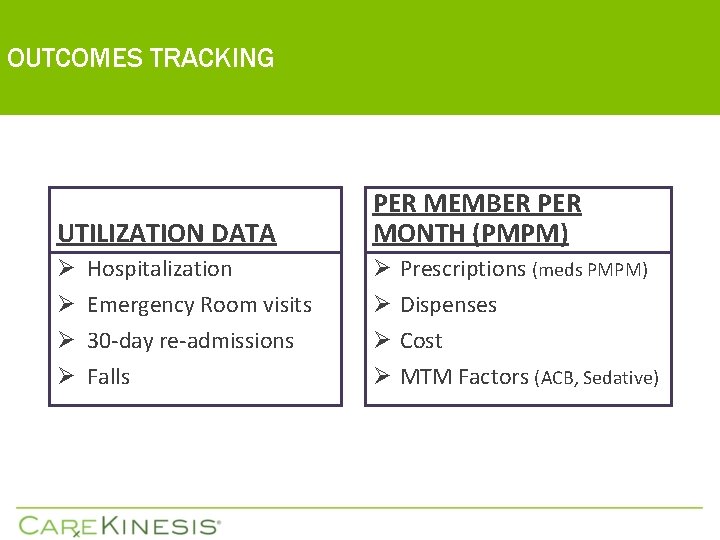
OUTCOMES TRACKING UTILIZATION DATA PER MEMBER PER MONTH (PMPM) Ø Hospitalization Ø Emergency Room visits Ø 30 -day re-admissions Ø Falls Ø Ø Prescriptions (meds PMPM) Dispenses Cost MTM Factors (ACB, Sedative)

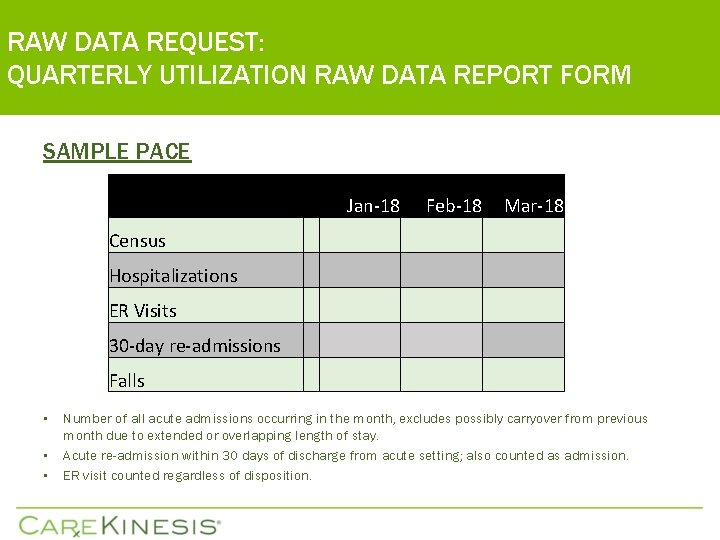
RAW DATA REQUEST: QUARTERLY UTILIZATION RAW DATA REPORT FORM SAMPLE PACE • • • Jan-18 Feb-18 Mar-18 Census Hospitalizations ER Visits 30 -day re-admissions Falls Number of all acute admissions occurring in the month, excludes possibly carryover from previous month due to extended or overlapping length of stay. Acute re-admission within 30 days of discharge from acute setting; also counted as admission. ER visit counted regardless of disposition.

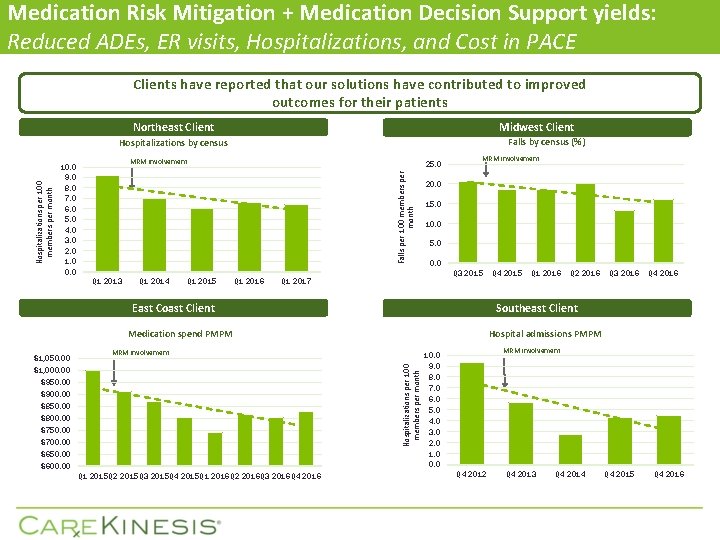
Medication Risk Mitigation + Medication Decision Support yields: Reduced ADEs, ER visits, Hospitalizations, and Cost in PACE Clients have reported that our solutions have contributed to improved outcomes for their patients Northeast Client Midwest Client Falls by census (%) MRM involvement 10. 0 9. 0 8. 0 7. 0 6. 0 5. 0 4. 0 3. 0 2. 0 1. 0 0. 0 25. 0 Falls per 100 members per month Hospitalizations by census Q 1 2013 Q 1 2014 Q 1 2015 Q 1 2016 MRM involvement 20. 0 15. 0 10. 0 5. 0 0. 0 Q 3 2015 Q 1 2017 East Coast Client Hospitalizations per 100 members per month $950. 00 $900. 00 $850. 00 $800. 00 $750. 00 $700. 00 $650. 00 $600. 00 Q 1 2015 Q 2 2015 Q 3 2015 Q 4 2015 Q 1 2016 Q 2 2016 Q 3 2016 Q 4 2016 Q 2 2016 Q 3 2016 Q 4 2016 Hospital admissions PMPM MRM involvement $1, 000. 00 Q 1 2016 Southeast Client Medication spend PMPM $1, 050. 00 Q 4 2015 MRM involvement 10. 0 9. 0 8. 0 7. 0 6. 0 5. 0 4. 0 3. 0 2. 0 1. 0 0. 0 Q 4 2012 Q 4 2013 Q 4 2014 Q 4 2015 Q 4 2016

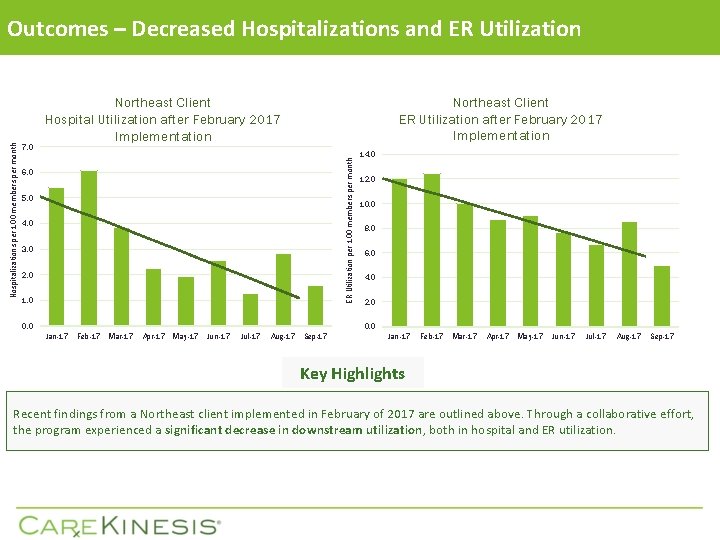
7. 0 Northeast Client ER Utilization after February 2017 Implementation Northeast Client Hospital Utilization after February 2017 Implementation ER Utilization per 100 members per month Hospitalizations per 100 members per month Outcomes – Decreased Hospitalizations and ER Utilization 6. 0 5. 0 4. 0 3. 0 2. 0 1. 0 0. 0 14. 0 12. 0 10. 0 8. 0 6. 0 4. 0 2. 0 0. 0 Jan-17 Feb-17 Mar-17 Apr-17 May-17 Jun-17 Jul-17 Aug-17 Sep-17 Key Highlights Recent findings from a Northeast client implemented in February of 2017 are outlined above. Through a collaborative effort, the program experienced a significant decrease in downstream utilization, both in hospital and ER utilization.

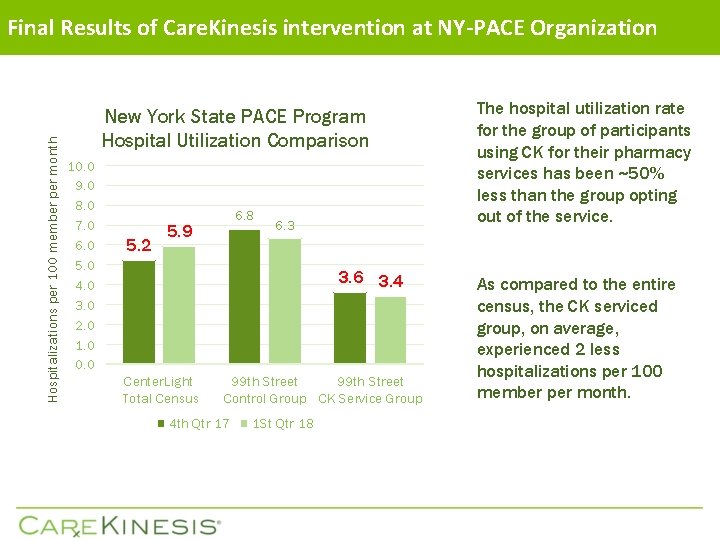
Hospitalizations per 100 member per month Final Results of Care. Kinesis intervention at NY-PACE Organization New York State PACE Program Hospital Utilization Comparison 10. 0 9. 0 8. 0 7. 0 6. 0 5. 0 4. 0 3. 0 2. 0 1. 0 0. 0 5. 2 6. 8 5. 9 6. 3 3. 6 3. 4 Center. Light Total Census 99 th Street Control Group CK Service Group 4 th Qtr 17 1 St Qtr 18 The hospital utilization rate for the group of participants using CK for their pharmacy services has been ~50% less than the group opting out of the service. As compared to the entire census, the CK serviced group, on average, experienced 2 less hospitalizations per 100 member per month.

CLINICAL INITIATIVE COLLABORATION OPPORTUNITIES OFFERINGS AT NO ADDITIONAL COST

STAR RATINGS AND QUALITY INDICATORS § QUALITY IS A FACTOR FOR VALUE-BASED OUTCOMES § SEVERAL STAR RATINGS AND QUALITY INDICATORS PERTAIN TO PHARMACY AND ARE EVIDENCE-BASED MEASURES. THESE ARE THE AREAS WHERE CAREKINESIS CAN ASSIST PACE ORGANIZATIONS.

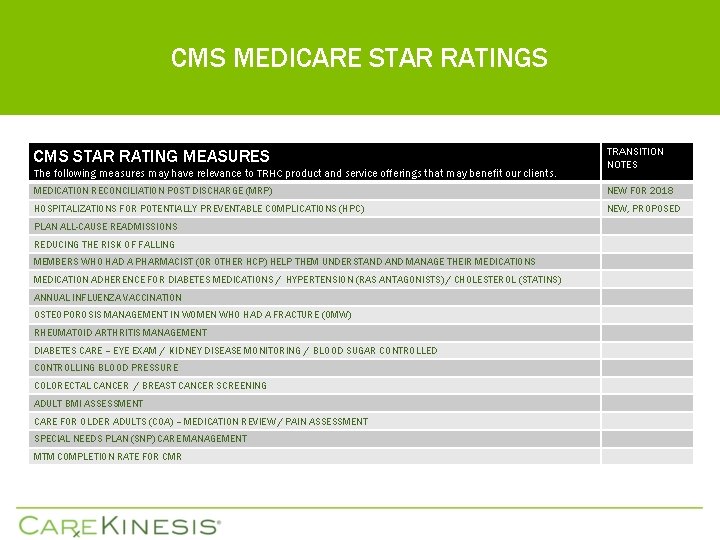
CMS MEDICARE STAR RATINGS CMS STAR RATING MEASURES The following measures may have relevance to TRHC product and service offerings that may benefit our clients. TRANSITION NOTES MEDICATION RECONCILIATION POST DISCHARGE (MRP) NEW FOR 2018 HOSPITALIZATIONS FOR POTENTIALLY PREVENTABLE COMPLICATIONS (HPC) NEW, PROPOSED PLAN ALL-CAUSE READMISSIONS REDUCING THE RISK OF FALLING MEMBERS WHO HAD A PHARMACIST (OR OTHER HCP) HELP THEM UNDERSTAND MANAGE THEIR MEDICATIONS MEDICATION ADHERENCE FOR DIABETES MEDICATIONS / HYPERTENSION (RAS ANTAGONISTS) / CHOLESTEROL (STATINS) ANNUAL INFLUENZA VACCINATION OSTEOPOROSIS MANAGEMENT IN WOMEN WHO HAD A FRACTURE (OMW) RHEUMATOID ARTHRITIS MANAGEMENT DIABETES CARE – EYE EXAM / KIDNEY DISEASE MONITORING / BLOOD SUGAR CONTROLLED CONTROLLING BLOOD PRESSURE COLORECTAL CANCER / BREAST CANCER SCREENING ADULT BMI ASSESSMENT CARE FOR OLDER ADULTS (COA) – MEDICATION REVIEW / PAIN ASSESSMENT SPECIAL NEEDS PLAN (SNP) CARE MANAGEMENT MTM COMPLETION RATE FOR CMR

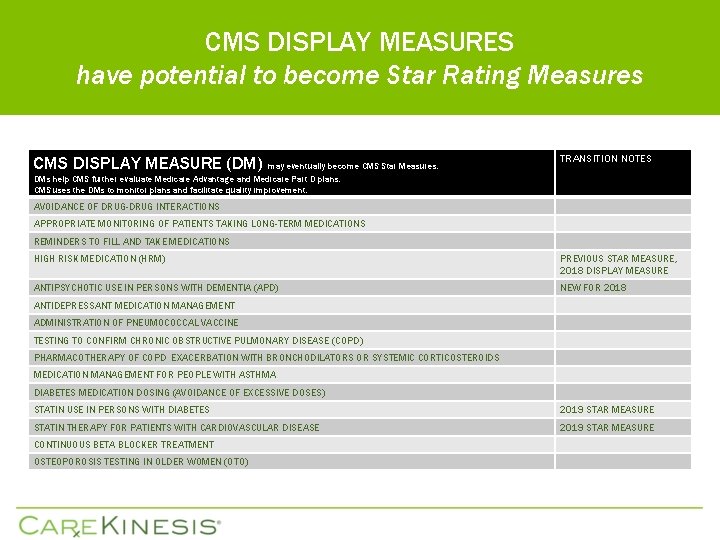
CMS DISPLAY MEASURES have potential to become Star Rating Measures CMS DISPLAY MEASURE (DM) may eventually become CMS Star Measures. TRANSITION NOTES DMs help CMS further evaluate Medicare Advantage and Medicare Part D plans. CMS uses the DMs to monitor plans and facilitate quality improvement. AVOIDANCE OF DRUG-DRUG INTERACTIONS APPROPRIATE MONITORING OF PATIENTS TAKING LONG-TERM MEDICATIONS REMINDERS TO FILL AND TAKE MEDICATIONS HIGH RISK MEDICATION (HRM) PREVIOUS STAR MEASURE, 2018 DISPLAY MEASURE ANTIPSYCHOTIC USE IN PERSONS WITH DEMENTIA (APD) NEW FOR 2018 ANTIDEPRESSANT MEDICATION MANAGEMENT ADMINISTRATION OF PNEUMOCOCCAL VACCINE TESTING TO CONFIRM CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) PHARMACOTHERAPY OF COPD EXACERBATION WITH BRONCHODILATORS OR SYSTEMIC CORTICOSTEROIDS MEDICATION MANAGEMENT FOR PEOPLE WITH ASTHMA DIABETES MEDICATION DOSING (AVOIDANCE OF EXCESSIVE DOSES) STATIN USE IN PERSONS WITH DIABETES 2019 STAR MEASURE STATIN THERAPY FOR PATIENTS WITH CARDIOVASCULAR DISEASE 2019 STAR MEASURE CONTINUOUS BETA BLOCKER TREATMENT OSTEOPOROSIS TESTING IN OLDER WOMEN (OTO)

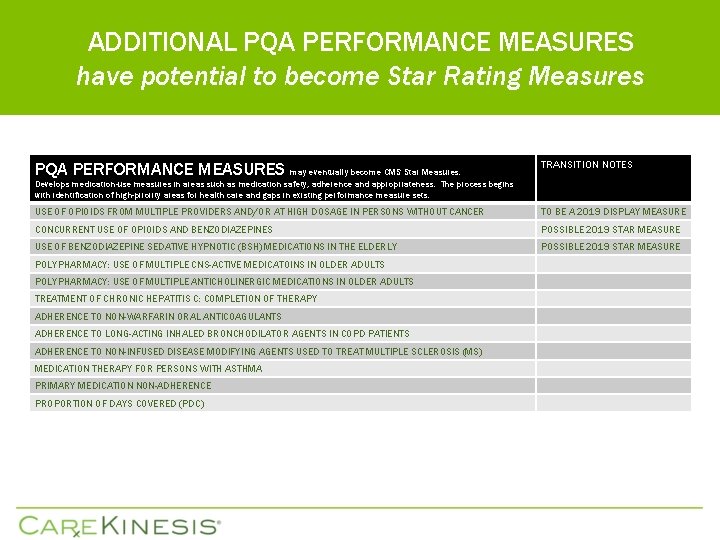
ADDITIONAL PQA PERFORMANCE MEASURES have potential to become Star Rating Measures PQA PERFORMANCE MEASURES may eventually become CMS Star Measures. TRANSITION NOTES Develops medication-use measures in areas such as medication safety, adherence and appropriateness. The process begins with identification of high-priority areas for health care and gaps in existing performance measure sets. USE OF OPIOIDS FROM MULTIPLE PROVIDERS AND/OR AT HIGH DOSAGE IN PERSONS WITHOUT CANCER TO BE A 2019 DISPLAY MEASURE CONCURRENT USE OF OPIOIDS AND BENZODIAZEPINES POSSIBLE 2019 STAR MEASURE USE OF BENZODIAZEPINE SEDATIVE HYPNOTIC (BSH) MEDICATIONS IN THE ELDERLY POSSIBLE 2019 STAR MEASURE POLYPHARMACY: USE OF MULTIPLE CNS-ACTIVE MEDICATOINS IN OLDER ADULTS POLYPHARMACY: USE OF MULTIPLE ANTICHOLINERGIC MEDICATIONS IN OLDER ADULTS TREATMENT OF CHRONIC HEPATITIS C: COMPLETION OF THERAPY ADHERENCE TO NON-WARFARIN ORAL ANTICOAGULANTS ADHERENCE TO LONG-ACTING INHALED BRONCHODILATOR AGENTS IN COPD PATIENTS ADHERENCE TO NON-INFUSED DISEASE MODIFYING AGENTS USED TO TREAT MULTIPLE SCLEROSIS (MS) MEDICATION THERAPY FOR PERSONS WITH ASTHMA PRIMARY MEDICATION NON-ADHERENCE PROPORTION OF DAYS COVERED (PDC)

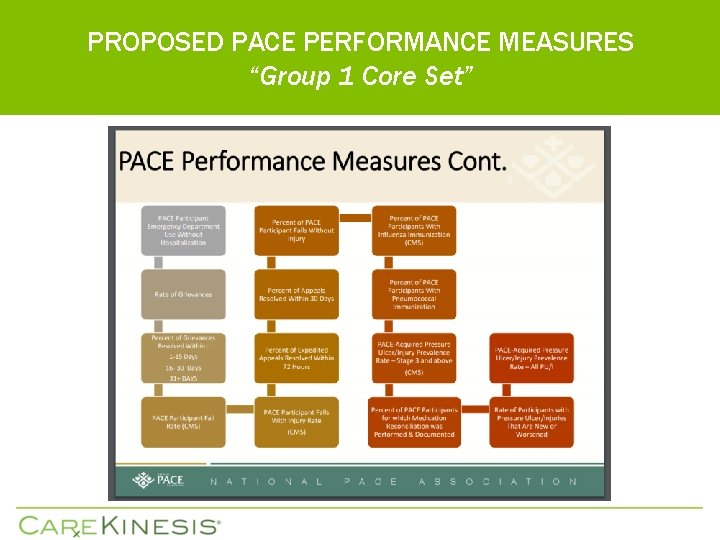
PROPOSED PACE PERFORMANCE MEASURES “Group 1 Core Set”

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES Are participants taking their medications as prescribed? Have excess medications been found in participant homes? Are participant falls related to medications? Is utilization creeping up and you don’t know why? CONSIDER PARTNERING IN AN EXISTING CLINICAL INITIATIVE OR LET’S COLLABORATE TO DESIGN ONE

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES § § § POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN § HCV THERAPY PROTOCOL § MEDICATION SAFETY REVIEWS § POLYPHARMACY CONFERENCE CALLS

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES • • • POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

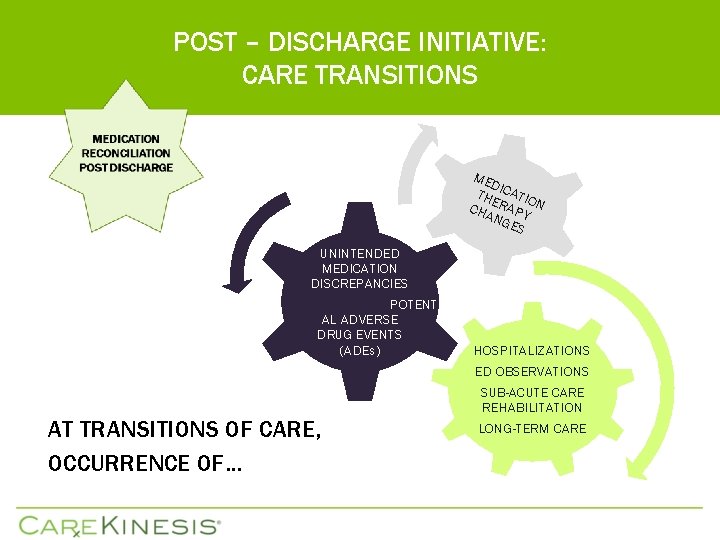
POST – DISCHARGE INITIATIVE: CARE TRANSITIONS ME D THE ICATIO CHA RAPY N NG ES UNINTENDED MEDICATION DISCREPANCIES POTENTI AL ADVERSE DRUG EVENTS (ADEs) HOSPITALIZATIONS ED OBSERVATIONS AT TRANSITIONS OF CARE, OCCURRENCE OF… SUB-ACUTE CARE REHABILITATION LONG-TERM CARE

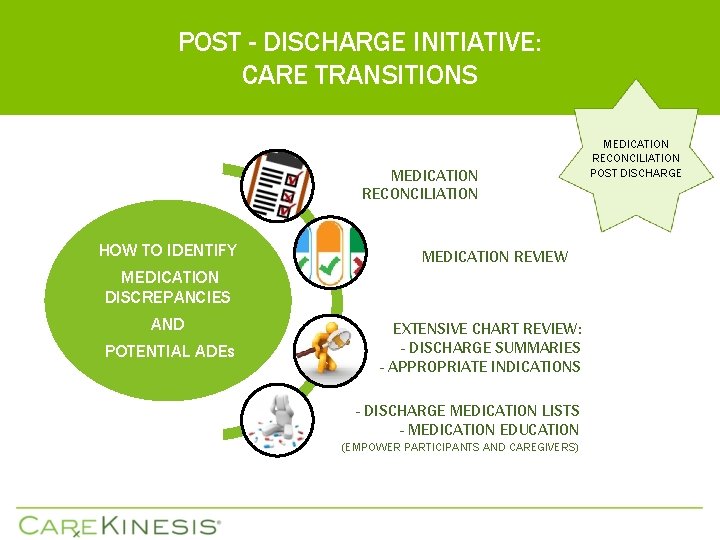
POST - DISCHARGE INITIATIVE: CARE TRANSITIONS MEDICATION RECONCILIATION HOW TO IDENTIFY MEDICATION DISCREPANCIES AND POTENTIAL ADEs MEDICATION REVIEW EXTENSIVE CHART REVIEW: - DISCHARGE SUMMARIES - APPROPRIATE INDICATIONS - DISCHARGE MEDICATION LISTS - MEDICATION EDUCATION (EMPOWER PARTICIPANTS AND CAREGIVERS) MEDICATION RECONCILIATION POST DISCHARGE

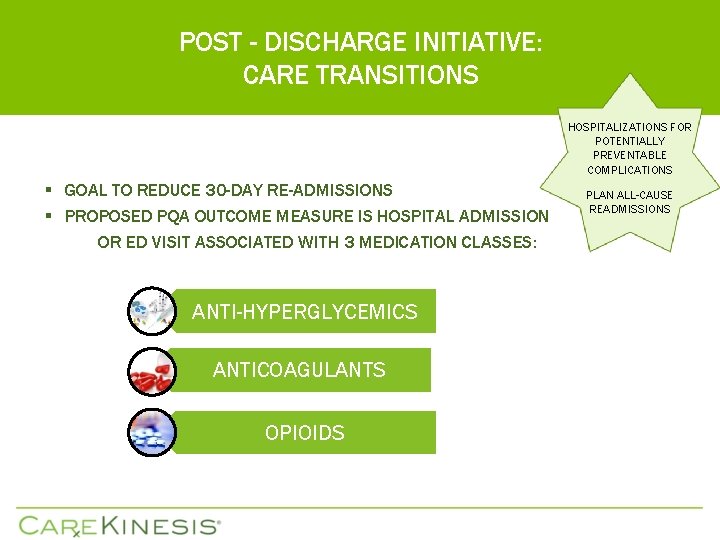
POST - DISCHARGE INITIATIVE: CARE TRANSITIONS HOSPITALIZATIONS FOR POTENTIALLY PREVENTABLE COMPLICATIONS § GOAL TO REDUCE 30 -DAY RE-ADMISSIONS § PROPOSED PQA OUTCOME MEASURE IS HOSPITAL ADMISSION OR ED VISIT ASSOCIATED WITH 3 MEDICATION CLASSES: ANTI-HYPERGLYCEMICS ANTICOAGULANTS OPIOIDS PLAN ALL-CAUSE READMISSIONS

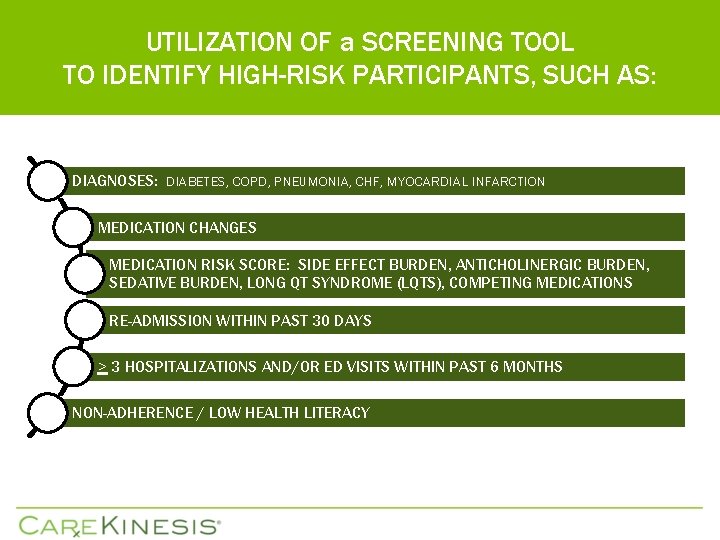
UTILIZATION OF a SCREENING TOOL TO IDENTIFY HIGH-RISK PARTICIPANTS, SUCH AS: DIAGNOSES: DIABETES, COPD, PNEUMONIA, CHF, MYOCARDIAL INFARCTION MEDICATION CHANGES MEDICATION RISK SCORE: SIDE EFFECT BURDEN, ANTICHOLINERGIC BURDEN, SEDATIVE BURDEN, LONG QT SYNDROME (LQTS), COMPETING MEDICATIONS RE-ADMISSION WITHIN PAST 30 DAYS > 3 HOSPITALIZATIONS AND/OR ED VISITS WITHIN PAST 6 MONTHS NON-ADHERENCE / LOW HEALTH LITERACY

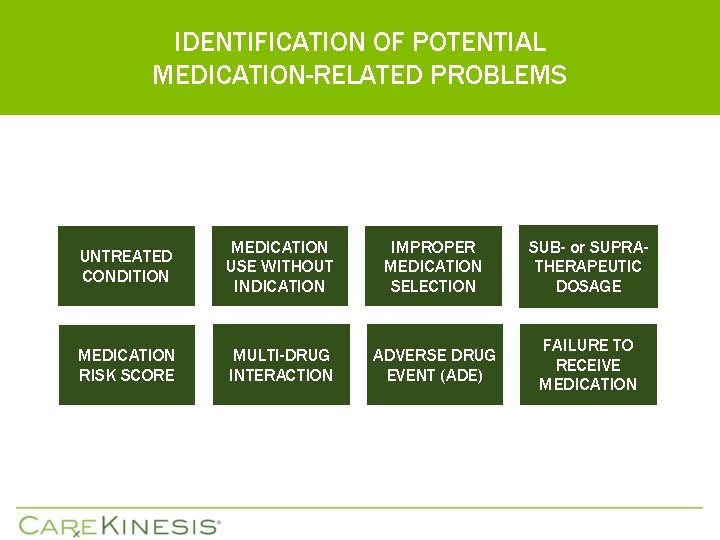
IDENTIFICATION OF POTENTIAL MEDICATION-RELATED PROBLEMS UNTREATED CONDITION MEDICATION USE WITHOUT INDICATION IMPROPER MEDICATION SELECTION SUB- or SUPRATHERAPEUTIC DOSAGE MEDICATION RISK SCORE MULTI-DRUG INTERACTION ADVERSE DRUG EVENT (ADE) FAILURE TO RECEIVE MEDICATION

TARGETED MEDICATION REVIEWS: TIMELY UPON POST - DISCHARGE TO IMPROVE OUTCOMES AND PREVENT RE-ADMISSION … q. PERFORM MEDICATION RECONCILIATION AND MEDICATION REVIEW AS CLOSE TO TIME OF DISCHARGE AS POSSIBLE § RECOMMEND MEDICATION THERAPY CHANGES AND MONITORING § IDENTIFICATION OF POTENTIAL MEDICATION-RELATED PROBLEMS § IMMUNIZATION SCREENING QUESTIONNAIRE q. PROVIDE MEDICATION AND CHRONIC DISEASE STATE EDUCATION TO EMPOWER PATIENTS AND CAREGIVERS (HEALTH LITERACY, ADHERENCE) § CONSIDER OUTREACH PHONE CALLS POST - DISCHARGE

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES • • • POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

FALL REDUCTION INITIATIVE REDUCING THE RISK OF FALLING q WHEN PERFORMING A FALLS ASSESSMENT, IT IS IMPORTANT TO CONSIDER THE MULTIPLE RISK FACTORS FOR FALLS MAY BE MODIFIABLE, SUCH AS CONTRIBUTING MEDICATIONS: § § § MEDICATION CHANGES WITHIN LAST 30 DAYS MEDICATION ASSOCIATED WITH FALLS HIGH AGGREGATE ANTICHOLINERGIC BURDEN HIGH AGGREGATE SEDATIVE BURDEN MULTI-DRUG INTERACTIONS, COMPETITIVE INHIBITION POTENTIAL ADVERSE EFFECTS q CLINICAL PHARMACISTS PERFORM TARGETED FALL ASSESSMENT MEDICATION REVIEWS, UPON REQUEST, AND PARTICIPATE IN FALLS MEETINGS, AS SCHEDULES PERMIT.

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES § • • § § § POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

APPROPRIATE PSYCHOTROPIC UTILIZATION ANTIPSYCHOTIC USE IN PERSONS WITH DEMENTIA q PREVIOUSLY ONLY LONG-TERM-CARE q NOW ALSO WILL INCLUDE COMMUNITY-DWELLING § In patients with behavioral and psychological symptoms of dementia (BPSD), antipsychotics have been associated with increased mortality risk due to cardiovascular (i. e. heart failure, CVA, sudden death) and infectious (i. e. pneumonia) complications, especially within the first 30 to 40 days of therapy initiation, as well as the potential adverse CNS effects. § As per the APA 2016 guidelines, a gradual dose reduction (GDR) is to be attempted within 4 months of therapy initiation. If GDR too rapid, may experience withdrawal symptoms that may be mistaken for behavioral disturbance. § If still clinically indicated, best practice is to document the actual behaviors associated with the dementia to assess efficacy of continued therapy. q CONSIDER UTILIZATION OF A CONSENT FORM FOR ANTIPSYCHOTIC USE IN BPSD

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES § § § § § POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

AIMM / Ho. Rd. E INITIATIVE FWA PREVENTION HOSPITALIZATIONS, RE-ADMISSIONS, MEDICATION ADHERENCE AND MANAGEMENT q WITHOUT A PROPER MEDICATION MANAGEMENT PROGRAM IN PLACE, NON-ADHERENCE COULD RESULT IN POOR CLINICAL AND ECONOMICAL OUTCOMES: § MEDICATION-RELATED HOSPITALIZATIONS § WASTEFUL SPENDING ON MEDICATIONS THAT PARTICIPANTS ARE NOT TAKING q IN-HOME OBSERVATIONAL ASSESSMENTS ARE BENEFICIAL TO EVALUATE MEDICATION TAKING BEHAVIOR AND IDENTIFY EXCESS MEDICATIONS UTILIZING ASSESSMENT TOOLS. q REMINDER ADHERENCE PACKAGING (MAC / MAP) q AUTOMATED MEDICATION DISPENSING SYSTEM (MEDACUBE)

Adherence Definition for Care. Kinesis q PARTICIPANTS MUST BE IN CONCORDANCE (IN AGREEMENT WITH PRESCRIBED THERAPY) TO BE ADHERENT.

NON-ADHERENCE? POTENTIAL HIGH-RISK PARTICIPANTS q. NUMBER OF MEDICATIONS q. DOSING FREQUENCY q. RECENT MEDICATION REGIMEN CHANGES q. HOSPITALIZATIONS / ED OBSERVATIONS q. CO-MORBIDITIES q. COGNITIVE IMPAIRMENT q. INDEPENDENT LIVING q. CAREGIVER STRESS

AIMM / HORDE Initiative q PARTICIPATION REQUEST FORM q TRAINING IS PROVIDED TO PACE STAFF § GENERALLY HOMECARE NURSES ADMINISTER THE ASSESSMENT § HOW TO PERFORM THE ADHERENCE ASSESSMENT AS WELL AS IDENTIFICATION AND DOCUMENTATION OF EXCESS MEDICATIONS

AIMM / Ho. Rd. E PROCESS PACE PARTICIPANTS: § if determined high-risk of non-adherence § if excess medications identified § Meda. Cube implementation PRE-PACE: § for all potential enrollees

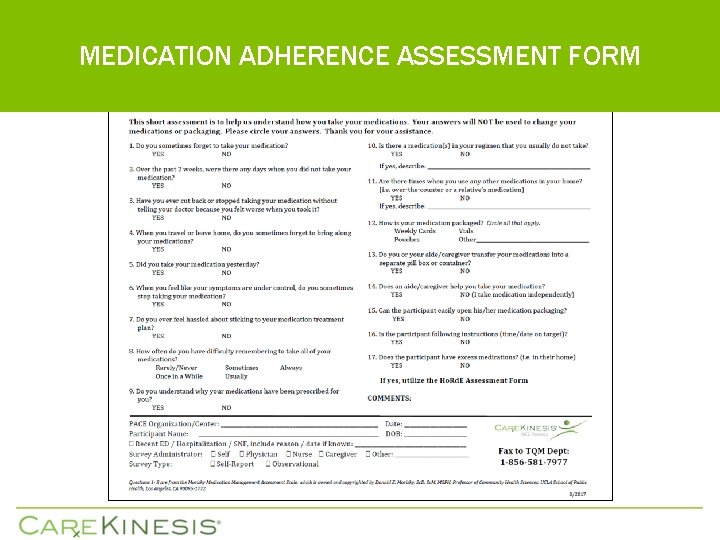
MEDICATION ADHERENCE ASSESSMENT FORM

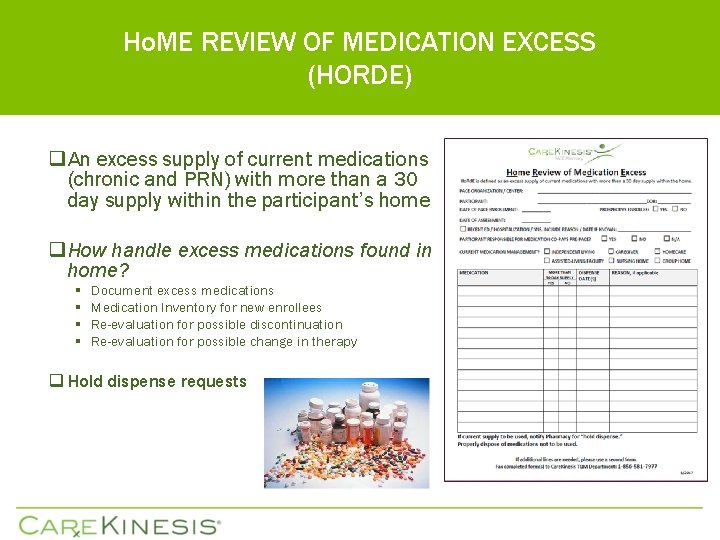
Ho. ME REVIEW OF MEDICATION EXCESS (HORDE) q An excess supply of current medications (chronic and PRN) with more than a 30 day supply within the participant’s home q How handle excess medications found in home? § § Document excess medications Medication Inventory for new enrollees Re-evaluation for possible discontinuation Re-evaluation for possible change in therapy q Hold dispense requests

A PERFECT FIT WITH THE ADHERENCE INITIATIVE: Considerations with Meda. Cube PRE - IMPLEMENTATION q Obtain “baseline” adherence assessment q Care. Kinesis will perform a targeted adherence medication review to potentially: reduce polypharmacy, consolidate dosing for ease of Meda. Cube use, avoidance of potential interactions and potentially improved adherence § 16 medication capacity (scheduled, as needed) § If administration time changes, may mitigate competitive inhibition interactions § Caution and close monitoring (vital signs, symptoms, etc)

A PERFECT FIT WITH THE ADHERENCE INITIATIVE: Considerations with Meda. Cube POST - IMPLEMENTATION q As participant becomes more adherent, clinical team may need to re-evaluate therapy and adjust doses q Perform follow-up adherence assessments q Obtain additional health outcome metrics (blood pressure, Hgb. A 1 C, lipid panel, hospitalizations, ED observations, re-admissions, etc)

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES § § § § § POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

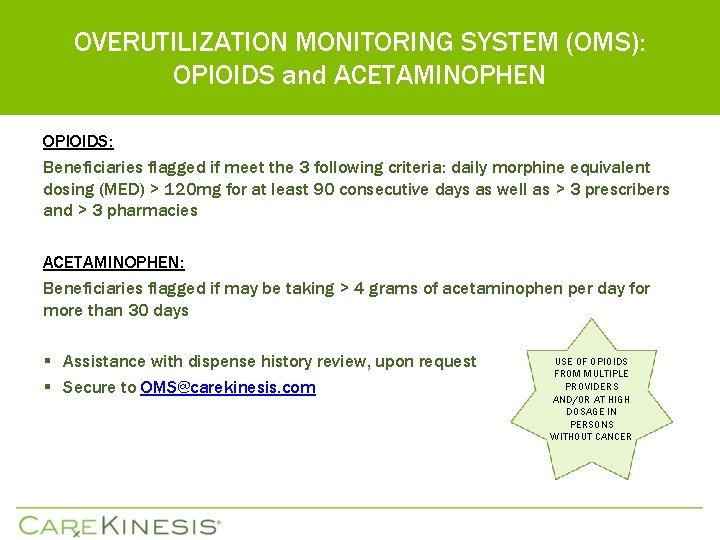
OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN OPIOIDS: Beneficiaries flagged if meet the 3 following criteria: daily morphine equivalent dosing (MED) > 120 mg for at least 90 consecutive days as well as > 3 prescribers and > 3 pharmacies ACETAMINOPHEN: Beneficiaries flagged if may be taking > 4 grams of acetaminophen per day for more than 30 days § Assistance with dispense history review, upon request § Secure to OMS@carekinesis. com USE OF OPIOIDS FROM MULTIPLE PROVIDERS AND/OR AT HIGH DOSAGE IN PERSONS WITHOUT CANCER

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES § § § § § POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

TREATMENT OF CHRONIC HEPATITIS C: COMPLETION OF THERAPY PROTOCAL DEVELOPMENT PURPOSE: INTENDED TO PROVIDE GUIDANCE TO PACE WITH PARTICIPANTS DIAGNOSED WITH HCV IN DETERMINING RISK ASSESSMENT (CANDIDATE), TREATMENT ALGORITHMS AND GUIDELINES ACCOMPANYING FORMS: HCV TREATMENT EXPECTATIONS AND MONITORING AVAILABLE ON THE EIRENERX PARTNER RESOURCES PAGE, CLINICAL RESOURCES

ACCOMPANYING FORMS: HCV TREATMENT EXPECTATIONS AND MONITORING

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES § § § § § POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

MEDICATION SAFETY REVIEWS - COMPREHENSIVE q Set up in advance q Participant list provided or may focus on highest risk participants (based on medication risk score) q CK Clinical team performs review of participants’ medications § § Polypharmacy High cost medications Multi-drug interactions, Competitive Inhibition interactions – administration times Aggregate Burdens – anticholinergic and sedative effects q Review with recommendations and rationale provided to Prescribers q Prescribers review and make medication changes, as appropriate

MEDICATION SAFETY REVIEWS - TARGETED q Set up in advance q Participant list provided, such as recurrent fallers concerned may be medication-related q CK Clinical team performs targeted review of participants’ medications q Review with recommendations and rationale provided to Prescribers q Prescribers review and make medication changes, as appropriate

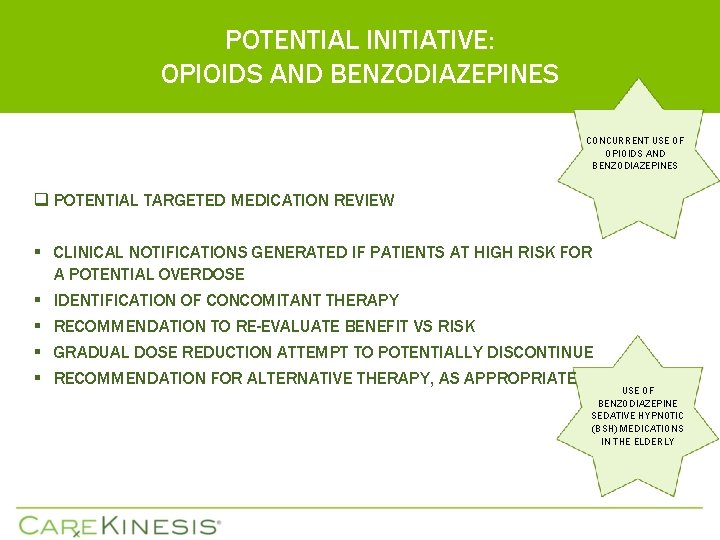
POTENTIAL INITIATIVE: OPIOIDS AND BENZODIAZEPINES CONCURRENT USE OF OPIOIDS AND BENZODIAZEPINES q POTENTIAL TARGETED MEDICATION REVIEW § CLINICAL NOTIFICATIONS GENERATED IF PATIENTS AT HIGH RISK FOR A POTENTIAL OVERDOSE § IDENTIFICATION OF CONCOMITANT THERAPY § RECOMMENDATION TO RE-EVALUATE BENEFIT VS RISK § GRADUAL DOSE REDUCTION ATTEMPT TO POTENTIALLY DISCONTINUE § RECOMMENDATION FOR ALTERNATIVE THERAPY, AS APPROPRIATE USE OF BENZODIAZEPINE SEDATIVE HYPNOTIC (BSH) MEDICATIONS IN THE ELDERLY

CLINICAL PERFORMANCE IMPROVEMENT INITIATIVES § § § § § POST - DISCHARGE INITIATIVE FALL REDUCTION INITIATIVE APPROPRIATE PSYCHOTROPIC UTILIZATION ADHERENCE INITIATIVE FOR MEDICATION MANAGEMENT (AIMM) HOME REVIEW OF MEDICATION EXCESS (Ho. Rd. E) OVERUTILIZATION MONITORING SYSTEM (OMS): OPIOIDS and ACETAMINOPHEN HCV THERAPY PROTOCOL MEDICATION SAFETY REVIEWS POLYPHARMACY CONFERENCE CALLS

POLYPHARMACY CONFERENCE CALLS q Set up in advance q Participant list provided or may focus on highest risk participants (based on medication risk score) q CK Clinical team performs review of participants’ medications § § Polypharmacy High cost medications Competitive Inhibition interactions – administration times Aggregate Burdens – anticholinergic and sedative effects q Prescribers and CK Clinical team discuss recommendations and rationale q Prescribers make medication changes, as appropriate

FINAL CONSIDERATIONS FOR VALUE-BASED OUTCOMES q MEDICATION RISK SCORE § Utilization: Identification of highest risk participants § Goal: Lower risk score to mitigate potential for adverse drug events (ADEs) q OUTCOMES WIZARD APPLICATION § Objectively captures instances of collaboration § Consideration and uptake of recommendations is essential § Improved health outcomes (i. e. hospitalizations, re-admissions, ED visits, falls)

LOOKING FORWARD TO COLLABORATION WITH YOUR CLIENT ORGANIZATION TO IMPROVE PARTICIPANT CARE PLEASE TELL US ABOUT YOUR CURRENT AND POTENTIAL QAPI INITIATIVES. THANK YOU.

CONTACTS Carlos Perez, MSN, RN-BC, Sr. VP, Total Quality Management E-Mail: CPerez@carekinesis. com Phone: 856 -840 -4831 Lisa Banks, Pharm. D, BCGP, VP, Clinical Initiatives E-Mail: LBanks@carekinesis. com Phone: 856 -840 -4852 Rachel Gilbert, CPh. T, Director, QAPI Email: RGilbert@carekinesis. com Phone: 856 -840 -4850 Heather Klarmann, CPh. T, QAPI Coordinator Email: HKlarmann@carekinesis. com Phone: 856 -242 -2548