Total Protein Measurement methods Human proteins More than









- Slides: 34

Total Protein Measurement methods

• Human proteins – More than 50, 000 • Within one cell 3000 to 5000 • Serum – More than 1400 different proteins



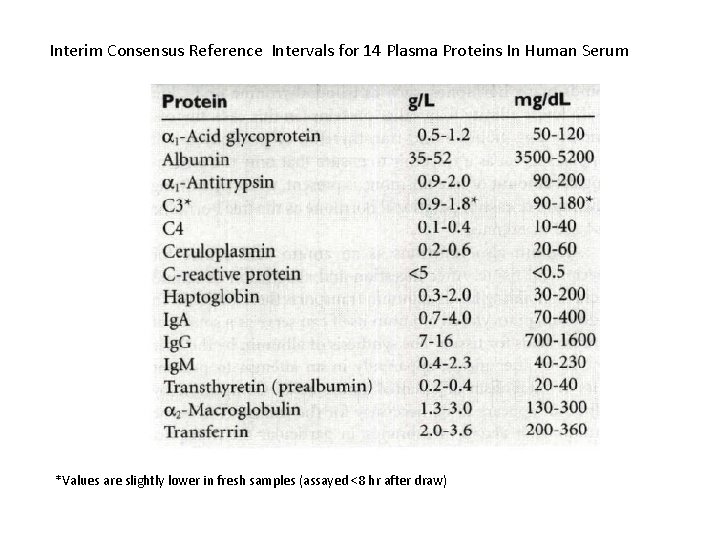
Interim Consensus Reference Intervals for 14 Plasma Proteins In Human Serum *Values are slightly lower in fresh samples (assayed <8 hr after draw)

• Distribution – Vascular, extravascular space – Kind and proportions of individual proteins • Molecular size • Specificity of some of their transport mechanisms • Disease

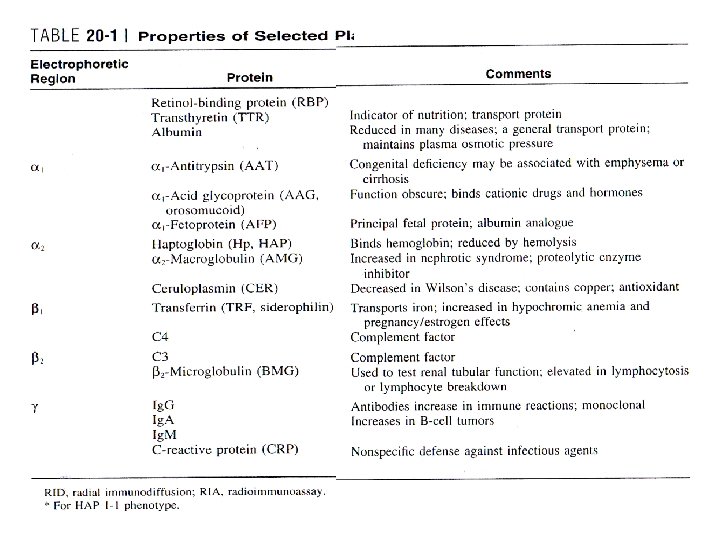
Plasma proteins • Alterations of plasma proteins – Genetic origin – Physiological – Pathological • Clinical findings • Technical

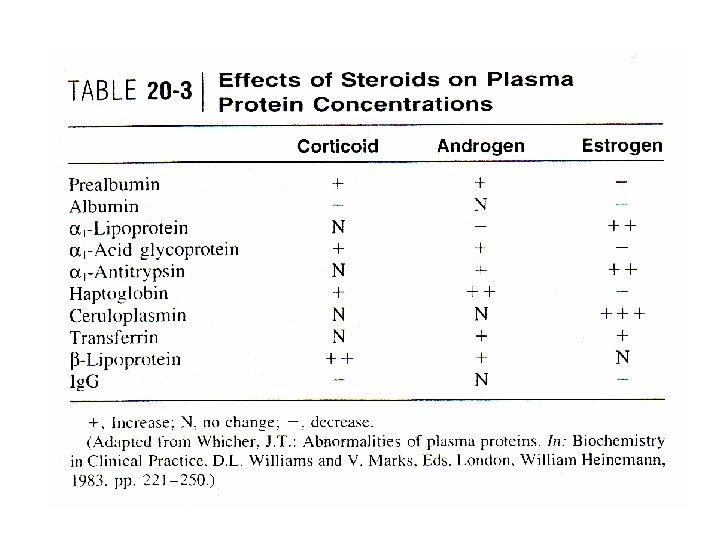
Plasma proteins • Patient ’s endocrine status – Rates of hepatic synthesis – Steroid hormones • Inflammatory acute-phase reaction • Mask effect


• Increased Plasma Levels – acute dehydration • no clinical utility • Synthetic rate and intravascular—extravascular shifts • Decreased Plasma Levels – Decreased synthesis • Primary or genetic – Analbuminemia • Acquired – Inflammatory processes – Increased catabolism • Utilization or loss

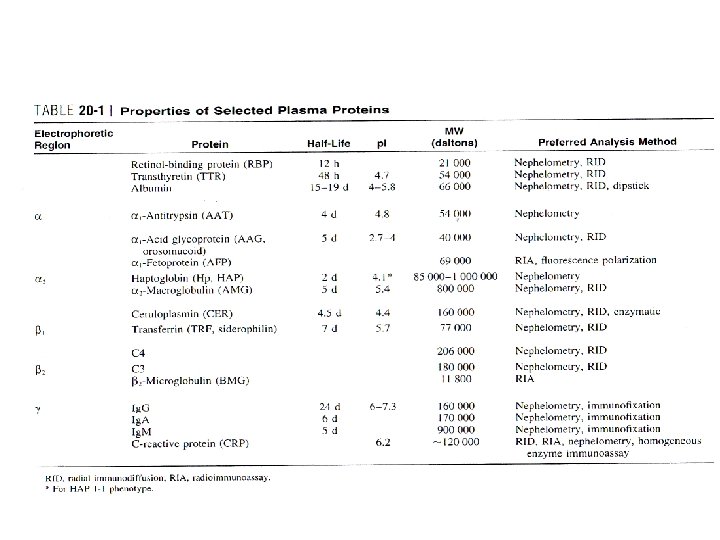
ANALYSIS OF PROTEINS Methods • Specific quantitative assays (Individual proteins) – Immunochemical methods • • • Nephelometry Turbidimetry RID Electroimmunoassay RIA or enzyme immunoassay (EIA) – very low concentrations • Detection and identification – Electrophoresis • Quantitative measurements of total protein

ANALYSIS OF PROTEINS Methods • Nephelometric and turbidimetric – Speed and ease – Formation of Ag-Ab complex – Light absorption & scattering

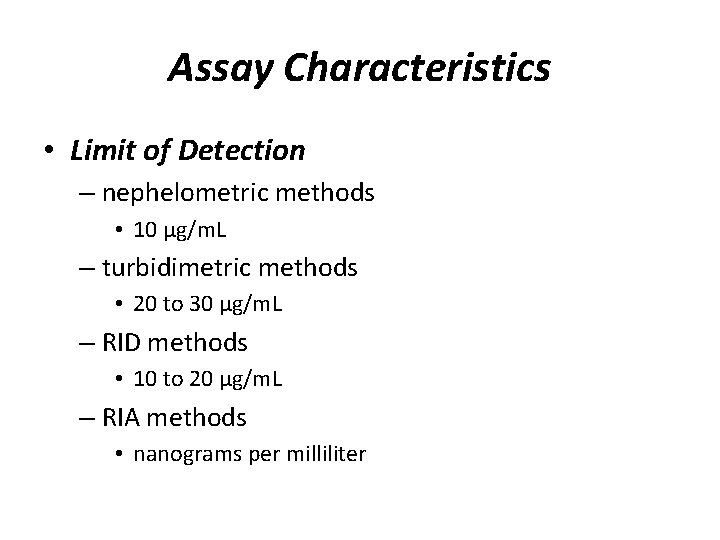
Assay Characteristics • Limit of Detection – nephelometric methods • 10 µg/m. L – turbidimetric methods • 20 to 30 µg/m. L – RID methods • 10 to 20 µg/m. L – RIA methods • nanograms per milliliter

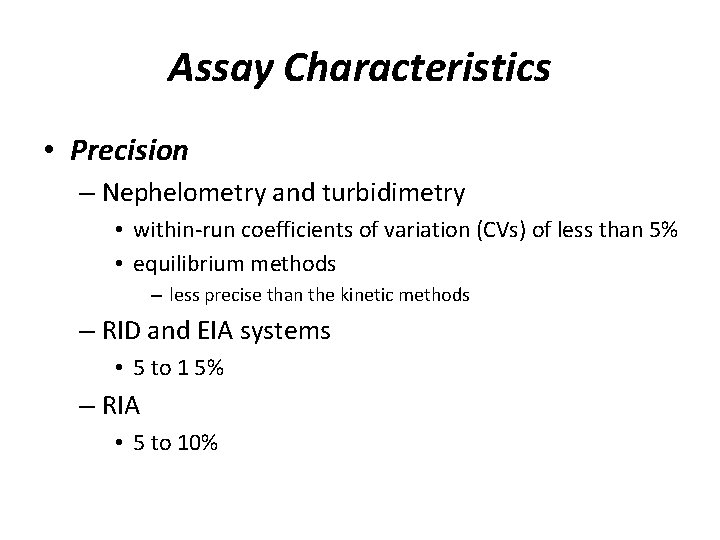
Assay Characteristics • Precision – Nephelometry and turbidimetry • within-run coefficients of variation (CVs) of less than 5% • equilibrium methods – less precise than the kinetic methods – RID and EIA systems • 5 to 1 5% – RIA • 5 to 10%

Assay Characteristics • Turnaround Time – Nephelometric and turbidimetric • Kinetic methods – Fast, within minutes • Equilibrium methods – Up to 1 h – RID • 24 to 48 h of incubation – RIA • Several hours

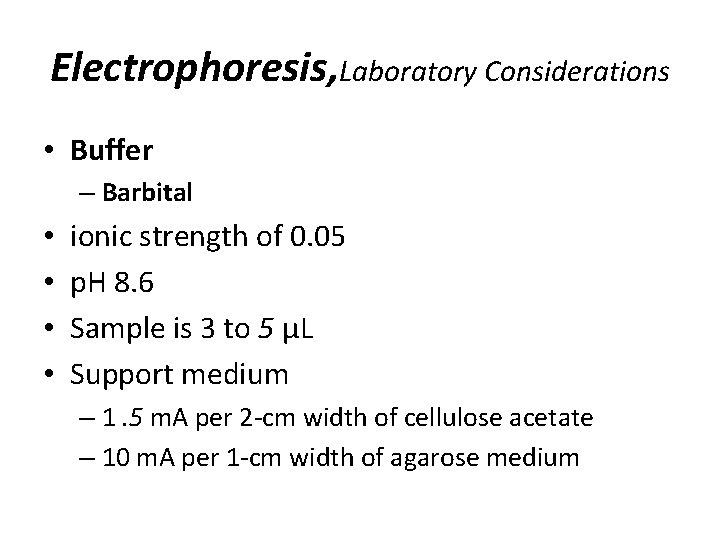
Electrophoresis, Laboratory Considerations • Buffer – Barbital • • ionic strength of 0. 05 p. H 8. 6 Sample is 3 to 5 µL Support medium – 1. 5 m. A per 2 -cm width of cellulose acetate – 10 m. A per 1 -cm width of agarose medium

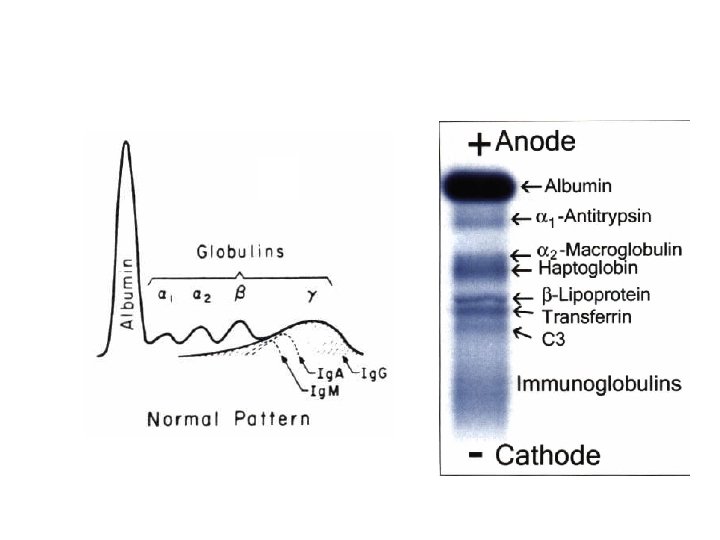
• Usually five bands (albumin, α 1, α 2, β and y) – a sixth band • Serum is fresh • buffer containing Ca 2+ ions • Densitometry – quantification of individual bands • Stain poorly – high proportions of lipid – Carbohydrate

• Mask effect – Too low concentrations – over- shadowed • Visualize bands – Amido black and Ponceau S – Coomassie brilliant blue • Dried, record • Lipoproteins – Migrate variable • Normal control serum


• Hyperproteinemia – Dehydration • Inadequate water intake • Excessive water loss – Vomiting, diarrhea, Addison’s disease, or diabetic acidosis • Hypoproteinemia – Hemodilution • Recumbent position – Decreases total protein concentration by 0. 3 to 0. 5 g. Id. L • All the individual plasma proteins to the same degree • Intravenous infusions

• Mild hyperproteinemia – Increases in APR and polyclonal immunoglobulins • Marked hyperproteinemia – high levels of the monoclonal immunoglobulins • Multiple myeloma

Methods (total protein) • Biuret Method • Peptide bonds react with Cu 2+ ions in alkaline solutions to form a colored product – Presence of peptide bonds • Tri-, oligo-, and polypeptides react – By spectrophotometry at 540 nm – The intensity of the color produced is proportional to the number of peptide bonds • Interference – Small peptides – Ammonium ions

Methods Biuret Method • Detection limit – 1 and 15 mg of protein • Simple, sufficiently precise • A fasting serum or plasma – to decrease lipemia • Hemolysis should be avoided

Direct Photometric Methods – Absorption of ultraviolet (UV) light – at 200 to 225 nm – at 270 to 290 nm • Limitations – Uneven distribution aromatic ring – Free tyrosine and tryptophan – Uric acid – Bilirubin

• At 200 to 225 nm – Peptide bonds are chiefly responsible for UV absorption • Removal of small interfering molecules – by dilution – gel filtration

Dye-Binding Methods • CBB binds to protonated amine groups of amino acid simple • Fast, & linear up to 150 mg/dl • Absorbance at 595 nm • Limitation – Unequal affinities for dyes – Binding capacities of individual proteins – Inability to define a consistent material for use as a calibrator

• Positive interferences – Tolbutamide , high concentrations of urea • Negative interferences – Very high concentrations of Na. Cl – Hydrogen chloride (HCl)

Fo. Iin-Ciocalteu (Lowry) Method • Reaction with Cu 2+ in alkaline solution to form copper—peptide bond protein complexes • Folin-Ciocalteu reagent – Phosphotungstic-phosphomolybdic acid • Tyrosine or tryptophan Reduce Cu 2+ • Reduced Cu 2+ form complex with Folin-Ciocalteu reagent – Measurement at 650 - 750 nm • Detection limit – 1 0— 60 mg/m. L

Fo. Iin-Ciocalteu (Lowry) Method • Measuring total protein in urine or CSF • Limitation – Positive interference • Drugs such as salicylates, chlorpromazine, tetracyclines, and some sulfa drugs – Removal of interferences • Gel filtration

Kjeldah. I’s Method • Acid digestion – Convert nitrogen in the protein to ammonium ion • Concentration of ammonia nitrogen – Double iodides (potassium and mercuric) form a colored complex with ammonia • Limitation – Time consuming • Impractical for wide spread routine use

Precipitation methods • Precipitation of protein – Sulfosalicylic acid – Trichloroacetic acid (TCA) • Scatter incident Light • TCA precipitation (Another approach) – Addition of biuret reagent to the precipitate • Suitable for a fairly large volume of specimen(urine)

Comments • Specimen Collection and Storage – Test specimens must be – Nonhemolyzed – Cell-free – Lipemic sera should not be assayed – Test tubes must remain covered • Dust and dirt particle contamination – Storage conditions – Use of outdated reagents

Calibration of Total Protein Methods • Reference material – Bovine or human albumin • Biuret method – Serum (or serum pool) with a normal albumin/globulin ratio • Precipitation methods • Dye-binding methods • Calculations – Calibration curve consisting of 8 to 15 points
