Topics 9 18 Acids and Bases Ionization of

Topics 9 & 18 Acids and Bases Ionization of Water The p. H Scale
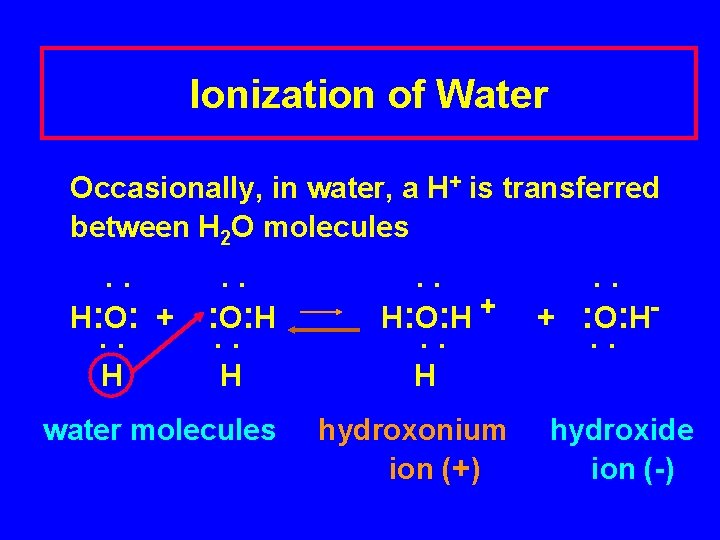
Ionization of Water Occasionally, in water, a H+ is transferred between H 2 O molecules . . H : O : + : O : H . . H water molecules . . H : O : H + . . + : O : H. . H hydroxonium ion (+) hydroxide ion (-)
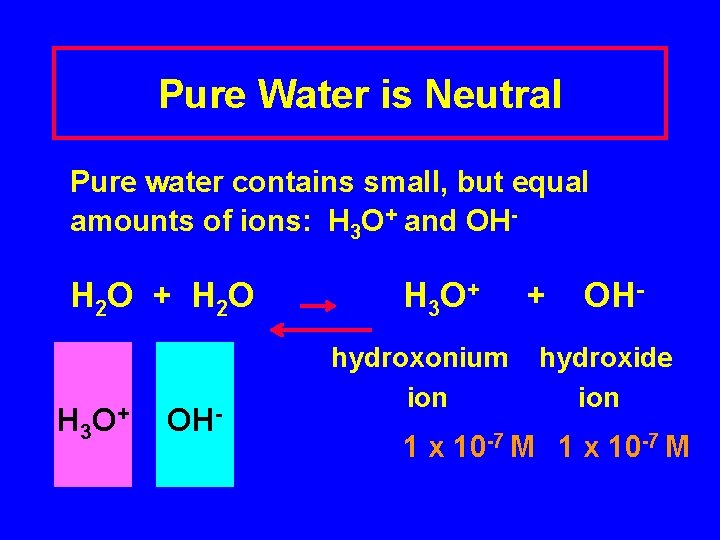
Pure Water is Neutral Pure water contains small, but equal amounts of ions: H 3 O+ and OH- H 2 O + H 2 O H 3 O + OH- H 3 O + hydroxonium ion + OH- hydroxide ion 1 x 10 -7 M
![Ion Product of Water Kw [ Kw ] = Molar concentration = [ H Ion Product of Water Kw [ Kw ] = Molar concentration = [ H](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-4.jpg)
Ion Product of Water Kw [ Kw ] = Molar concentration = [ H 3 O+ ] [ OH- ] = [ 1 x 10 -7 ] = 1 x 10 -14

Acids l Increase H+ l HCl (g) + H 2 O (l) H 3 O+ (aq) + Cl- (aq) l More [H 3 O+] than water > 1 x 10 -7 M l As H 3 O+ increases, OH- decreases [H 3 O+] > [OH-] H 3 O + OH-

Bases l. Increase the hydroxide ions (OH-) H 2 O l. Na. OH (s) Na+(aq) + OH- (aq) l. More [OH-] than water, [OH-] > 1 x 10 -7 M l When OH- increases, H 3 O+ decreases [OH ] > [H 3 O+] H 3 O + OH-
![Using Kw The [OH- ] of a solution is 1. 0 x 10 - Using Kw The [OH- ] of a solution is 1. 0 x 10 -](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-7.jpg)
Using Kw The [OH- ] of a solution is 1. 0 x 10 - 3 M. What is the [H 3 O+]? Kw = [H 3 O+ ] [OH- ] [H 3 O+] = 1. 0 x 10 -14 [OH-] [H 3 O+] = 1. 0 x 10 -14 1. 0 x 10 - 3 = 1. 0 x 10 -14 = 1. 0 x 10 -11 M
![Learning Check p. H 1 The [H 3 O+] of lemon juice is 1. Learning Check p. H 1 The [H 3 O+] of lemon juice is 1.](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-8.jpg)
Learning Check p. H 1 The [H 3 O+] of lemon juice is 1. 0 x 10 -3 M. What is the [OH-] of the solution? 1) 1. 0 x 103 M 2) 1. 0 x 10 -11 M 3) 1. 0 x 1011 M
![Solution p. H 1 The [H 3 O+] of lemon juice is 1. 0 Solution p. H 1 The [H 3 O+] of lemon juice is 1. 0](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-9.jpg)
Solution p. H 1 The [H 3 O+] of lemon juice is 1. 0 x 10 - 3 M. What is the [OH-]? [OH- ] = 1. 0 x 10 -14 1. 0 x 10 - 3 = 1. 0 x 10 -11 M
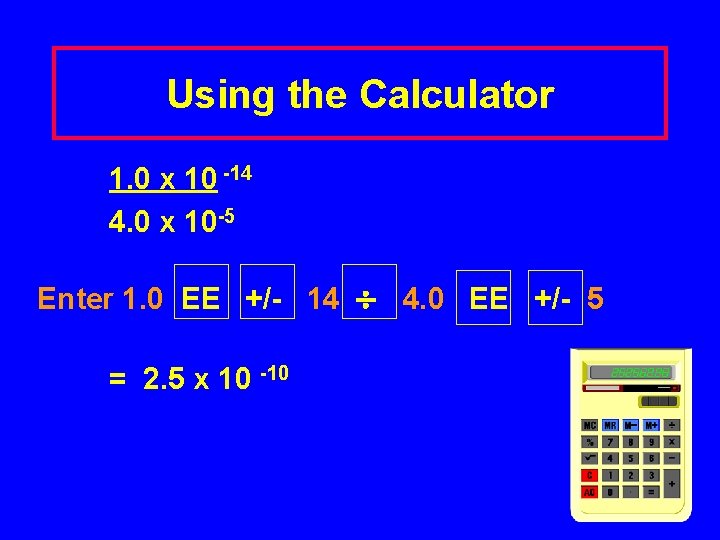
Using the Calculator 1. 0 x 10 -14 4. 0 x 10 -5 Enter 1. 0 EE +/- 14 4. 0 EE +/- 5 = 2. 5 x 10 -10
![Learning Check p. H 2 The [OH-] of a solution is 5 x 10 Learning Check p. H 2 The [OH-] of a solution is 5 x 10](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-11.jpg)
Learning Check p. H 2 The [OH-] of a solution is 5 x 10 -5 M. What is the [H 3 O+ ] of the solution? 1) 2 x 10 - 5 M 2) 1 x 1010 M 3) 2 x 10 -10 M
![Solution p. H 2 The [OH-] of a water solution is 5 x 10 Solution p. H 2 The [OH-] of a water solution is 5 x 10](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-12.jpg)
Solution p. H 2 The [OH-] of a water solution is 5 x 10 -5 M. What is the [H 3 O+] in the solution? [ H 3 O+] = 1. 0 x 10 -14 5 x 10 - 5 On some calculators: 1. 0 EE +/- 14 5 EE +/- 5 = 2 x 10 -10 M
![Learning Check p. H 3 A. The [OH-] when [H 3 O+ ] of Learning Check p. H 3 A. The [OH-] when [H 3 O+ ] of](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-13.jpg)
Learning Check p. H 3 A. The [OH-] when [H 3 O+ ] of 1 x 10 - 4 M 1) 1 x 10 -6 M 2) 1 x 10 -8 M 3) 1 x 10 -10 M B. The [H 3 O+] when [OH- ] of 5 x 10 -9 M 1) 1 x 10 - 6 M 2) 2 x 10 - 6 M 3) 2 x 10 -7 M
![Solution p. H 3 Kw = [H 3 O+ ][OH-] = 1. 0 x Solution p. H 3 Kw = [H 3 O+ ][OH-] = 1. 0 x](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-14.jpg)
Solution p. H 3 Kw = [H 3 O+ ][OH-] = 1. 0 x 10 14 A. (3) [OH- ] = 1. 0 x 10 -14 = 1. 0 x 10 -10 1. 0 x 10 - 4 B. (2) [H 3 O+] = 1. 0 x 10 -14 = 2 x 10 - 6 5 x 10 - 9
![p. H l Indicates the acidity [H 3 O+] of the solution l p. p. H l Indicates the acidity [H 3 O+] of the solution l p.](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-15.jpg)
p. H l Indicates the acidity [H 3 O+] of the solution l p. H = - log [H 3 O+] l From the French pouvoir hydrogene (“hydrogen power” or power of hydrogen)
![p. H In the expression for [H 3 O+] 1 x 10 -exponent the p. H In the expression for [H 3 O+] 1 x 10 -exponent the](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-16.jpg)
p. H In the expression for [H 3 O+] 1 x 10 -exponent the exponent = p. H [H 3 O+] = 1 x 10 -p. H M
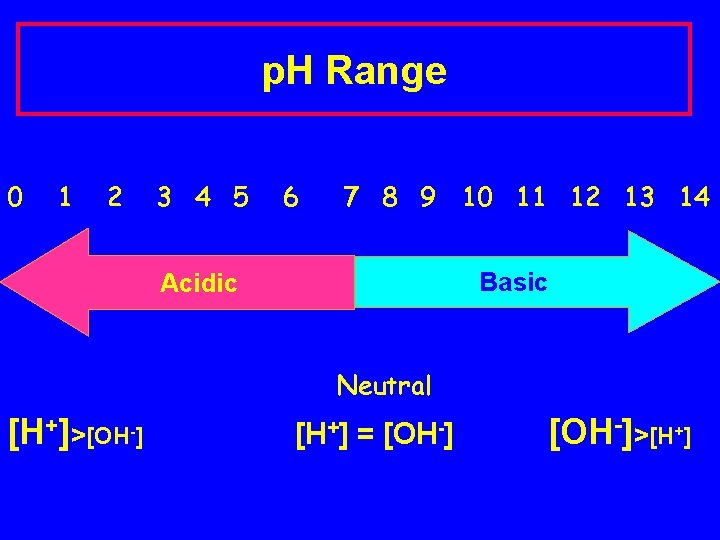
p. H Range 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Basic Acidic Neutral [H+]>[OH-] [H+] = [OH-]>[H+]
![Some [H 3 O+] and p. H [H 3 O+] p. H 1 x Some [H 3 O+] and p. H [H 3 O+] p. H 1 x](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-18.jpg)
Some [H 3 O+] and p. H [H 3 O+] p. H 1 x 10 -5 M 5 1 x 10 -9 M 9 1 x 10 -11 M 11
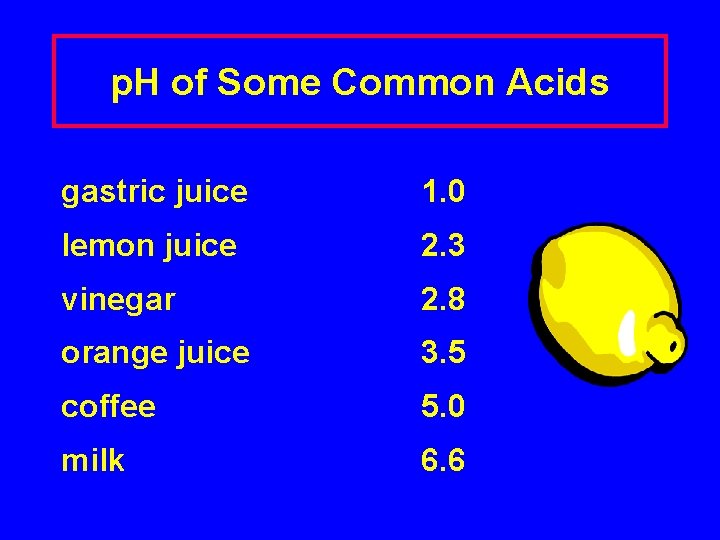
p. H of Some Common Acids gastric juice 1. 0 lemon juice 2. 3 vinegar 2. 8 orange juice 3. 5 coffee 5. 0 milk 6. 6

p. H of Some Common Bases blood 7. 4 tears 7. 4 seawater 8. 4 milk of magnesia 10. 6 household ammonia 11. 0
![Learning Check p. H 4 A. The [H 3 O+] of tomato juice is Learning Check p. H 4 A. The [H 3 O+] of tomato juice is](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-21.jpg)
Learning Check p. H 4 A. The [H 3 O+] of tomato juice is 1 x 10 -4 M. What is the p. H of the solution? 1) - 4 2) 4 3) 8 B. The [OH-] of an ammonia solution is 1 x 10 -3 M. What is the p. H of the solution? 1) 3 2) 11 3) -11
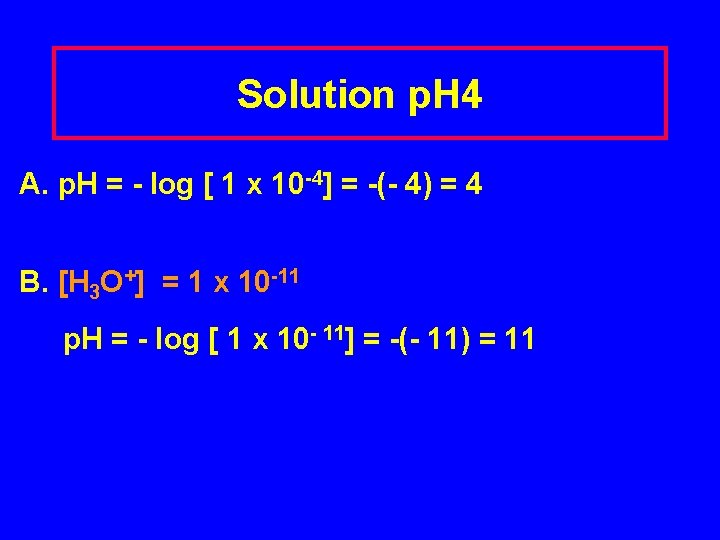
Solution p. H 4 A. p. H = - log [ 1 x 10 -4] = -(- 4) = 4 B. [H 3 O+] = 1 x 10 -11 p. H = - log [ 1 x 10 - 11] = -(- 11) = 11

Learning Check p. H 5 The p. H of a soap is 10. What is the [H 3 O+] of the soap solution? 1) 1 x 10 - 4 M 2) 1 x 1010 M 3) 1 x 10 - 10 M

Solution p. H 5 The p. H of a soap is 10. What is the [H 3 O+] of the soap solution? [H 3 O+] = 1 x 10 -p. H M = 1 x 10 -10 M
![p. H on the Calculator [H 3 O+] is 4. 5 x 10 -6 p. H on the Calculator [H 3 O+] is 4. 5 x 10 -6](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-25.jpg)
p. H on the Calculator [H 3 O+] is 4. 5 x 10 -6 M p. H = 4. 5 x EXP(or EE) 6+/- LOG +/= 5. 35
![Learning Check p. H 6 A soap solution has a [H 3 O+] = Learning Check p. H 6 A soap solution has a [H 3 O+] =](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-26.jpg)
Learning Check p. H 6 A soap solution has a [H 3 O+] = 2 x 10 -8 M. What is the p. H of the solution? 1) 8 2) 7. 7 3) 6
![Solution p. H 6 A soap solution has a [H 3 O+] = 2. Solution p. H 6 A soap solution has a [H 3 O+] = 2.](http://slidetodoc.com/presentation_image_h2/e04dd75959ffc7f404e67f7e6302b0a2/image-27.jpg)
Solution p. H 6 A soap solution has a [H 3 O+] = 2. 0 x 10 -8 M. What is the p. H of the solution? B) 2. 0 EE 8 +/- LOG +/- = 7. 7
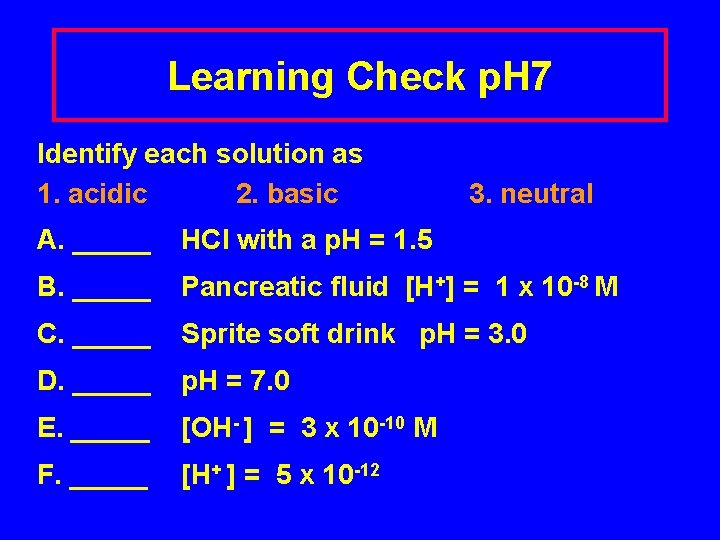
Learning Check p. H 7 Identify each solution as 1. acidic 2. basic 3. neutral A. _____ HCl with a p. H = 1. 5 B. _____ Pancreatic fluid [H+] = 1 x 10 -8 M C. _____ Sprite soft drink p. H = 3. 0 D. _____ p. H = 7. 0 E. _____ [OH- ] = 3 x 10 -10 M F. _____ [H+ ] = 5 x 10 -12

Solution p. H 7 Identify each solution as 1. acidic 2. basic 3. neutral A. _1__ HCl with a p. H = 1. 5 B. _2__ Pancreatic fluid [H+] = 1 x 10 -8 M C. _1__ Sprite soft drink p. H = 3. 0 D. _3__ p. H = 7. 0 E. _1__ [OH-] = 3 x 10 -10 M F. _2__ [H+] = 5 x 10 -12
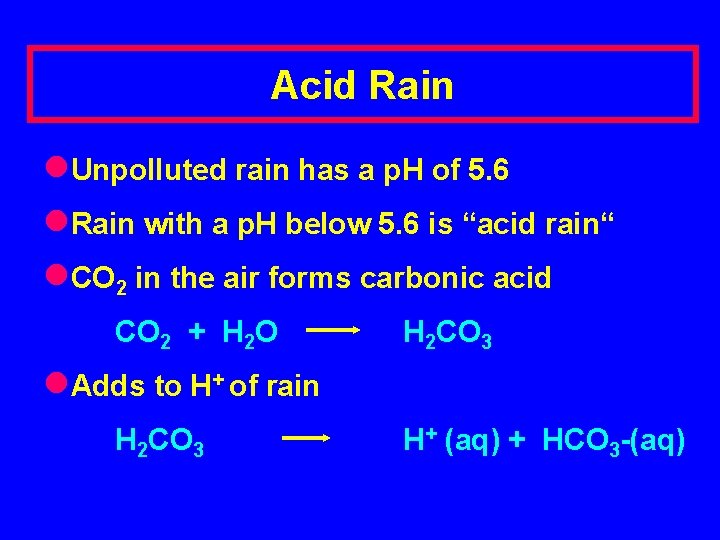
Acid Rain l. Unpolluted rain has a p. H of 5. 6 l. Rain with a p. H below 5. 6 is “acid rain“ l. CO 2 in the air forms carbonic acid CO 2 + H 2 O H 2 CO 3 l. Adds to H+ of rain H 2 CO 3 H+ (aq) + HCO 3 -(aq)

Sources of Acid Rain l. Power stations l. Oil refineries l. Coal with high S content l. Car and truck emissions l. Bacterial decomposition, and lighting hitting N 2

SO 2 NO and NO 2 26 million tons in 1980 22 million tons in 1980 Mt. St Helens (1980) 400, 000 tons SO 2 l Reactions with oxygen in air form SO 3 2 SO 2 + O 2 2 SO 3 l Reactions with water in air form acids SO 3 + H 2 O H 2 SO 4 sulphuric acid NO + H 2 O HNO 2 nitrous acid HNO 2 + H 2 O HNO 3 nitric acid

Effects of Acid Rain l Leaches Al from soil, which kills fish l Fish kills in spring from runoff due to accumulation of large amounts of acid in snow l Dissolves waxy coatings that protect leaves from bacteria l Corrodes metals, textiles, paper and leather
- Slides: 33