TOPICAL FLUORIDES INTRODUCTION o Fluorides have been proved

TOPICAL FLUORIDES

INTRODUCTION o Fluorides have been proved to the single most effective weapon in our limited arsenal of anticaries agents. o Dental profession has also been cognizant of the limitations of fluoridation of community water supply. o In 1941 began the era of topical fluorides. o In India >70% of population cannot be covered by water fluoridation.

o The term topical fluoride therapy refers to the use of systems containing relatively large concentrations of fluoride that are applied locally or topically, to erupted tooth surfaces to prevent the formation of dental caries. o This term encompasses the use of the fluoride rinses, dentifrices, pastes, gel and solution that are applied in various manners. - Haris and Garcia Goday.

RATIONALE o Is to speed the rate and increase the concentration of fluoride acquisition above the level which occurs naturally. o Best time to apply topical fluorides is soon after eruption. o Periodic applications of fluoride would enable vulnerable enamel sites that are partially demanerialized to accumulate fluoride.

RATIONALE o Bibby in 1942 was the first to demonstrate that the repeated application of sodium or potassium fluoride to teeth of children significantly reduced their caries prevalence.

PROFESSIONALLY APPLIED FLUORIDES o Dental personnel have been applying fluoride to the teeth since the early 1940’s. o Currently recognized agents include various fluoride solutions , gels and varnishes.

SODIUM FLUORIDE o In 1940 Volker et al showed invitro that the solubility of enamel could be appreciably reduced by treating it with a fluoride solution. o Milestone studies were conducted by Bibby (1941) and Knutson (1942, 1947, 1948).

METHOD OF PREPARATION OF SODIUM FLUORIDE o 2% Na. F solution can be prepared by dissolving 20 g of powder in 1 liter of distilled water in a plastic bottle.

METHOD OF APPLICATION OF SODIUM FLUORIDE – KNUTSON’S TECHNIQUE. o Cleaning and polishing of the teeth. o Opposing quadrants are isolated with cotton rolls and teeth are dried thoroughly. o 2% Na. F is applied with cotton applicator and dried on the teeth for 4 min. o Procedure is repeated for remaining quadrants.

METHOD OF APPLICATION OF SODIUM FLUORIDE – KNUTSON’S TECHNIQUE. ¨ Patient is instructed to avoid eating, drinking or rinsing for 30 minutes. ¨ 2 nd, 3 rd and 4 th applications are given at weekly intervals. ¨ A full series of 4 treatments is recommended at ages 3, 7, 11 and 13 years.

MECHANISM OF ACTION OF Na. F SOLUTION. o It reacts with HA crystals to form Ca. F 2. o Due to the high concentration of fluoride in 2% Na. F to which the solubility product of Ca. F 2 get excluded fast and the initial reaction is followed by drastic reduction in its rate and the phenomenon is called “Choking Off” o Further calcium fluoride reacts with HA to form fluoridated HA.

ADVANTAGES o Relatively stable when kept in a plastic container. o Taste is well accepted, non irritating and does not cause discoloration of tooth structure. o Multiple chair procedures in public health programmes. o The series of treatments must be repeated only 4 times rather than at annual or semiannual intervals.
DISADVATANGES o The major disadvantage is that the patient must make 4 visits to the dentist within a relatively short time.

STANNOUS FLUORIDE o Muhler et al in 1947 found that stannous fluoride to be 3 times more effective than sodium fluoride

METHOD OF PREPARATION OF STANNOUS FLUORIDE o Freshly prepared before each use. o Gelatin capsules are priorly filled with 0. 8 g powdered stannous fluoride and are stored. o Just before application, the content of one capsule is dissolved in 10 ml of distilled water in a plastic container.

METHOD OF APPLICATION OF STANNOUS FLUORIDE BY MUHLER o Thorough prophylaxis. o Isolation of teeth o Quadrant or half mouth treated at one time. o Applied with cotton applicators, and kept for 4 minutes. o Recommended frequency is once/year.

MECHANISM OF ACTION OF STANNOUS FLUORIDE o Stannous fluoride with HA in addition to fluoride, the tin of stannous fluoride also reacts with enamel and a new crystalline product formed that is stannous-tri-flurophosphate which is more resistant to decay than enamel. o Tin hydroxy phosphate is also formed and is responsible for metallic taste.
ADVANTAGES o The procedure frequency complies with one year recall appointment schedule.

DISADVANTAGES o Material is not stable in aqueous solutions. o Since 8% solution is quite astringent and disagreeable in taste. o Occasionally causes a reversible tissue irritation. o Pigmentation of teeth.

ACIDULATED PHOSPHATE FLUORIDE o In 1963 Brudevold et al found out that phosphate containing fluoride acid solution was of maximum beneficial effect.

PRACTICAL DIFFICULTIES WITH THE SOLUTION o Teeth must be kept wet with solution for 4 minutes. o APF solution is acidic, sour and bitter in taste. o Repeated applications necessitates the use of suction. o Multiple chair programme and expensive. o To overcome all these problems APF gels were introduced.

ACIDULATED PHOSPHATE FLUORIDE APF gel 1. Relatively costly APF solution 1. Relatively cheaper 2. Readily available (imported in India) 2. Prepared easily 3. Self application is possible 3. Applied by dentist / Auxiliary staff

Preparation of ACIDULATED PHOSPHATE FLUORIDE o Contains 1. 23% fluoride in 0. 1 M phosphoric acid at a p. H of 3 o Prepared by dissolving 20 mg of Na. F in liter of 0. 1 M phosphoric acid. o To this is added 50% hydrofluoride to adjust to p. H at 3 and F concentration to 1. 23% o For the preparation of APF gel, a gelling agent methylcellulose or Hydroxyethyl cellulose is to be added.
Method of application of ACIDULATED PHOSPHATE FLUORIDE o Thorough prophylaxis o Teeth are isolated o APF solution is applied and the teeth are kept moist for 4 minutes. o Floss may be used for inter proximal areas. o Semiannual applications o Gel can be used as self applications using trays.

Mechanism of action o Initially leads to dehydration and shrinkage in the volume of HAP crystals which further on hydrolysis forms Dicalcium phosphate dihydrate (DCPD). This leads to formation of FA. o For deeper penetration, a continuous supply of fluoride is required. So APF has to be applied every 30 seconds and teeth be kept wet for 4 mins.

ADVANTAGES o Requires only 2 applications in a year and is thus suited for most dental office routines. o The gel can be self applied and thus the cost of application also gets reduced.

DISADVANTAGES o Teeth should be kept wet for 4 min which increases chair-side time. o Acidic, sour and bitter in taste. o Cannot be stored in glass containers. o Causes surface alterations of many restorative materials.
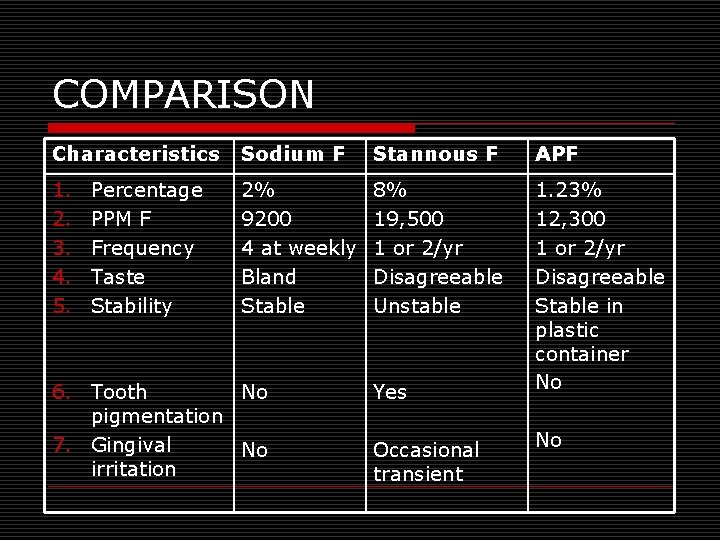
COMPARISON Characteristics Sodium F Stannous F APF 1. 2. 3. 4. 5. 2% 9200 4 at weekly Bland Stable 8% 19, 500 1 or 2/yr Disagreeable Unstable 1. 23% 12, 300 1 or 2/yr Disagreeable Stable in plastic container No Percentage PPM F Frequency Taste Stability 6. Tooth No pigmentation 7. Gingival No irritation Yes Occasional transient No

MONOFLUOROPHOSPHATE o Fluoride atom is covalently bonded to phosphorous atom. o It is unique because only fluorapatite forms even when it is applied to enamel in high concentration or at low p. H.
FLUORIDE VARNISHES o Fluoride retention in enamel was found to be enhanced by sealing the surface of teeth with 2 – ethyl – 2 cyanoarylate after fluoride applications.
FLUORIDE VARNISHES o 2 most commonly used varnishes are: 1. Duraphat (2. 26% F) 2. Fluorprotector (0. 7%)

COMPOSITION OF FLUORIDE VARNISH o Duraphat: - Natural resin. - A neutral colophomium base, containing 5% wt Na. F (2. 26% F) dissolved in ethanol. - Banana flavoured and sets on tooth surface in a yellow – brown film.

COMPOSITION OF FLUORIDE VARNISH o Fluorprotector: - polyurethane based transparent resin, containing 0. 1% Fluoride as difluorosilane (0. 9%wt) dissolved in ethyl acetate and isoamyl propionate solution.

COMPOSITION OF FLUORIDE VARNISH o Biflurid: - contains 2. 7% weight as sodium fluoride and 2. 92% as calcium fluoride. o Carex: - contains 1. 8% of Na. F o Durafluor - 5% Na. F in a alcoholic suspension of natural resins o Fluoritop marketed in India.

COMPOSITION OF FLUORIDE VARNISH o CAVITY SHIELD: - Most recent. - 5% Na. F in a resinous base. Advantages: - avoids wastage. - prevents over application. - reduces chance of over ingestion.

TECHNIQUE OF VARNISH APPLICATION o Prophylaxis o Teeth dried but not isolated with cotton. o A total of 0. 3 -0. 5 ml of varnish equivalent to 6. 9 -11. 5 mg F is required to cover the full dentition. o Applied on lower arch first o Wait for 4 min o Not to rinse or drink anything for 1 hour and not to eat anything solid upto 18 hours.

Mechanism of action o When applied, a reservoir of F ions gets build up around the enamel of the teeth. o From this F keeps on slowly releasing and continuously reacting with HA crystals. o A part of calcium fluoride so formed in low concentration further reacts with crystals of HAP and forms FAP.

Clinical considerations o Patients who appear to be at high risk to caries: manifested by bacteriologic findings or development of several new cavities or areas of demineralization, xerostomia caused by medications, metabolic disturbances such as sjogren’s syndrome or radiation treatment.

RECOMMENDATIONS 1. Caries active individuals 2. Children shortly after periods of tooth eruption, especially those who are not caries free. 3. Those on medication or have received radiation. 4. After periodontal surgery 5. Patients with fixed or removable prosthesis. 6. Patient with eating disorder. 7. Mentally and physically challenged individuals.

SELF APPLIED TOPICAL FLUORIDES

FLUORIDE DENTIFRICES o 1955 – introduced for sale over the counter. o The most commonly evaluated are: - sodium fluoride - stannous fluoride - sodium monoflurophosphate - organic amine fluoride

DENTIFRICES CONTAINING MONOFLUOROPHOSPHATE o Preferred chemical form of fluoride o Since 1969 o Does not occur in nature o In recent formulations aluminium oxide abrasive have been used.

FLUORIDE MOUTHRINSES o First described by Bibby in 1946 o Most widely used caries preventive public health methods o ADA acceptance in 1975 for neutral Na. F and APF mouthrinses. o Later stannous fluoride was also accepted.

Sodium fluoride mouthrinses o 0. 2% Na. F for weekly use or 0. 05% Na. F for daily use. o Intended to use by forcefully swishing 10 ml of the liquid around the mouth for 60 sec before expectorating it. o Preparation: by dissolving 200 mg of Na. F tablet (10 mg Na. F and the rest lactose as filler) in 5 teaspoons of fresh clean water (approx 25 ml) o Caries reduction was less than 25 – 30%.

Recommendations for fluoride mouthrinses o Patients in fluoride deficient areas. o A swish and swallow technique should be followed if the concentration of drinking water is 0. 3 ppm or less. o Beneficial for patients with increased caries risk e. g. : for those undergoing orthodontic treatment as well as patients under radiotherapy.

FLUORIDE GELS o Fluoride gels for self application include neutral sodium fluoride and APF with a fluoride concentration of 5, 000 ppm and stannous fluoride which has a conc of 1000 ppm. o Applied in trays or brushed on teeth. o Patient should be cautioned to expectorate. o Not recommended for children 6 years and younger.

FLUORIDE TOXICITY

o Used in excessive quantities, fluorides can produce toxic and even lethal outcomes when ingested, inhaled or absorbed into the body. o Probable toxic dose (PTD): defined as the dose of ingested fluoride that should trigger immediate therapeutic intervention and hospitalization because of the likelihood of serious toxic consequences.

o Adults: CLD=32 to 64 mg of fluoride/kg bodywt 320 mg for 10 kg child STD= 8 to 16 mg F/kg body wt.
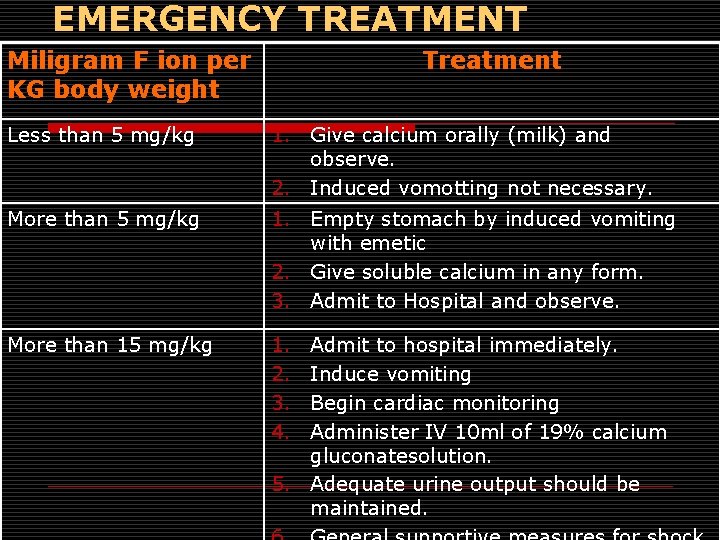
EMERGENCY TREATMENT Miligram F ion per KG body weight Treatment Less than 5 mg/kg 1. Give calcium orally (milk) and observe. 2. Induced vomotting not necessary. More than 5 mg/kg 1. Empty stomach by induced vomiting with emetic 2. Give soluble calcium in any form. 3. Admit to Hospital and observe. More than 15 mg/kg 1. 2. 3. 4. Admit to hospital immediately. Induce vomiting Begin cardiac monitoring Administer IV 10 ml of 19% calcium gluconatesolution. 5. Adequate urine output should be maintained.
Acute toxicity o Symptoms: v v Gastrointestinal Neurological CVS Blood chemistry

Chronic toxicity o Results from long term ingestion of fluoride. Ø Dental fluorosis Ø Skeletal fluorosis

q Dental fluorosis is caused by excessive intake of fluoride during tooth development. q Ingestion of fluoride concentration 2 or 3 times greater than the recommended amount caused white flecks and chalky opaque areas on the tooth enamel (mild fluorosis). q Consumption of water containing 4 times the recommended amount of fluoride causes a brown pitted corroded appearance on the enamel surface.

Skeletal fluorosis o Known as knock knee syndrome. o Osteosclerosis, ossification of tendons, pain and stiffness of joints, outward bending of legs, continuous back pain.

- Slides: 55