Topic2 History of the Nature of Matter History











- Slides: 18

Topic#2: History of the Nature of Matter

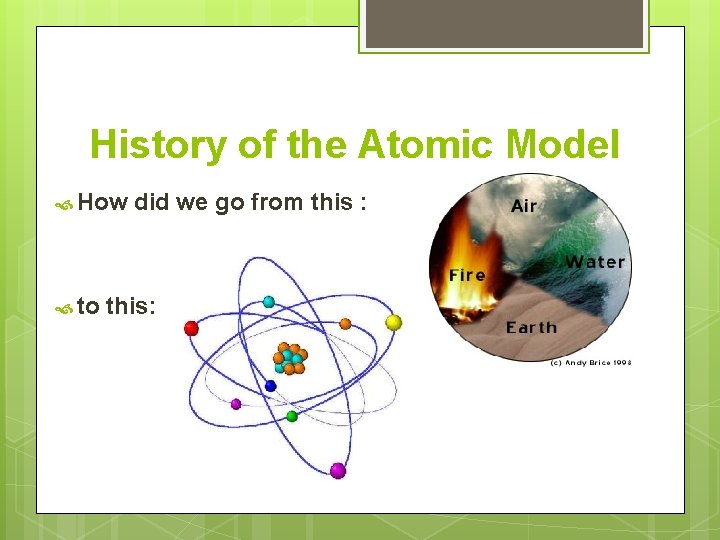
History of the Atomic Model How to did we go from this : this:

Empedocles (450 BCE) Empedocles proposed that matter was made of 4 elements: Earth Fire Water Air

Democritus : (400 BCE) Democritus suggested matter was made up of tiny particles that could not be broken down further. He called these “Atomos”

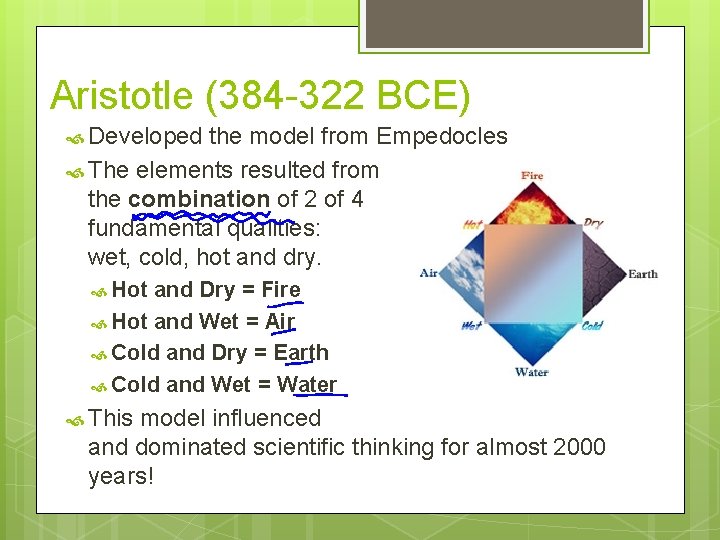
Aristotle (384 -322 BCE) Developed the model from Empedocles The elements resulted from the combination of 2 of 4 fundamental qualities: wet, cold, hot and dry. Hot and Dry = Fire Hot and Wet = Air Cold and Dry = Earth Cold and Wet = Water This model influenced and dominated scientific thinking for almost 2000 years!

Alchemists: (500 CEto 1500 AD) Alchemists were the first people to perform hands on experimentations. They were part philosopher, mystic, magician and chemists. They believed some elements could change into others. Ex. Change base metals into gold They also wanted to find a substance that could give them eternal life and a universal solvent that would dissolve all substances.

Sir Francis Bacon (1600’s) One of the first scientists to develop new knowledge as a result of experiments. The Industrial Revolution started so telescopes, microscopes, gauges etc. were being invented.

Robert Boyle (1626 – 1691) Robert Boyle believed in the Greek philosophers “Four Elements” but said it needed to be worked on. He recognized that elements could be combined to form compounds.

Antoine de Lavoisier (1743 – 1794) He defined “elements” as pure substances that cannot be decomposed (broken down) into simpler substances. He discovered 23 elements. He recognized that mixtures existed and identified air as a mixture of oxygen and other gases.

Henry Cavendish (1731 – 1810) Cavendish experimented by mixing metal with acid which produced a flammable gas (hydrogen). He discovered that his gas would burn in oxygen and produce water. This showed that water is a compound not an element!

John Dalton (early 1800 s) Dalton described atoms as “tiny billiard balls”. Dalton’s Atomic Theory: All matter is made up of small particles called atoms Atoms cannot be created, destroyed or divided into smaller parts

John Dalton (early 1800 s) Dalton’s Atomic Theory: All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements Compounds are created when atoms of different elements link together in definite proportions

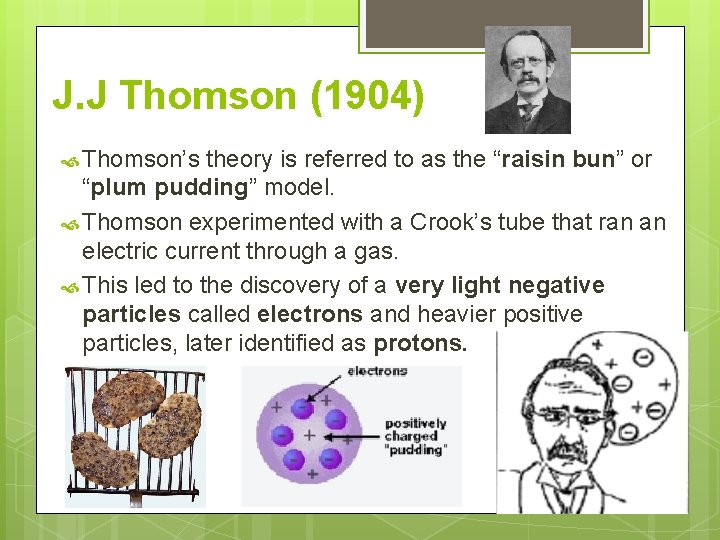
J. J Thomson (1904) Thomson’s theory is referred to as the “raisin bun” or “plum pudding” model. Thomson experimented with a Crook’s tube that ran an electric current through a gas. This led to the discovery of a very light negative particles called electrons and heavier positive particles, later identified as protons.

J. J Thomson (1904) This disproved Dalton’s theory that atoms are indivisible. Thompson proposed: Electrons have a small mass and a negative charge An atom is a sphere of positive electricity Negative electrons are embedded in the positive sphere, so that the resulting atom is neutral or uncharged

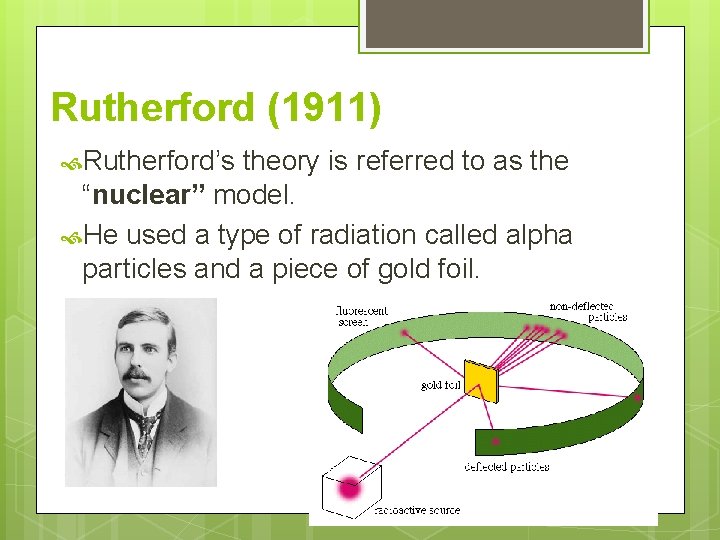
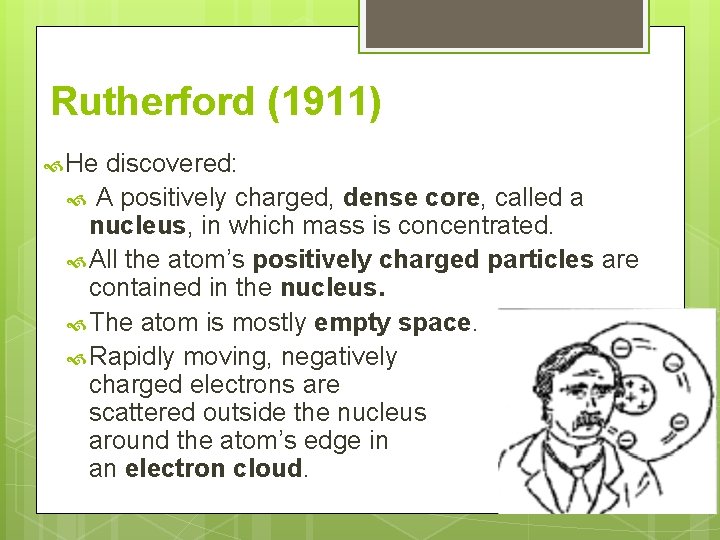
Rutherford (1911) Rutherford’s theory is referred to as the “nuclear” model. He used a type of radiation called alpha particles and a piece of gold foil.

Rutherford (1911) He discovered: A positively charged, dense core, called a nucleus, in which mass is concentrated. All the atom’s positively charged particles are contained in the nucleus. The atom is mostly empty space. Rapidly moving, negatively charged electrons are scattered outside the nucleus around the atom’s edge in an electron cloud.

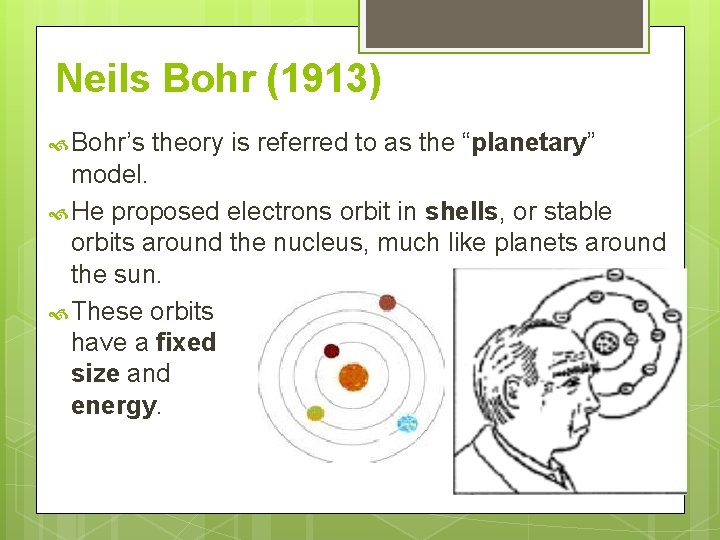
Neils Bohr (1913) Bohr’s theory is referred to as the “planetary” model. He proposed electrons orbit in shells, or stable orbits around the nucleus, much like planets around the sun. These orbits have a fixed size and energy.

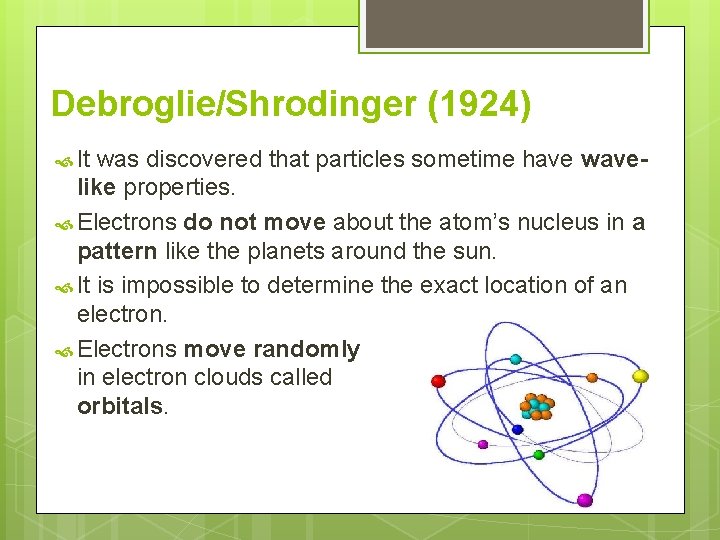
Debroglie/Shrodinger (1924) It was discovered that particles sometime have wavelike properties. Electrons do not move about the atom’s nucleus in a pattern like the planets around the sun. It is impossible to determine the exact location of an electron. Electrons move randomly in electron clouds called orbitals.