Topic Vapor Pressure Boiling vs Evaporation BOILING occurs

Topic: Vapor Pressure
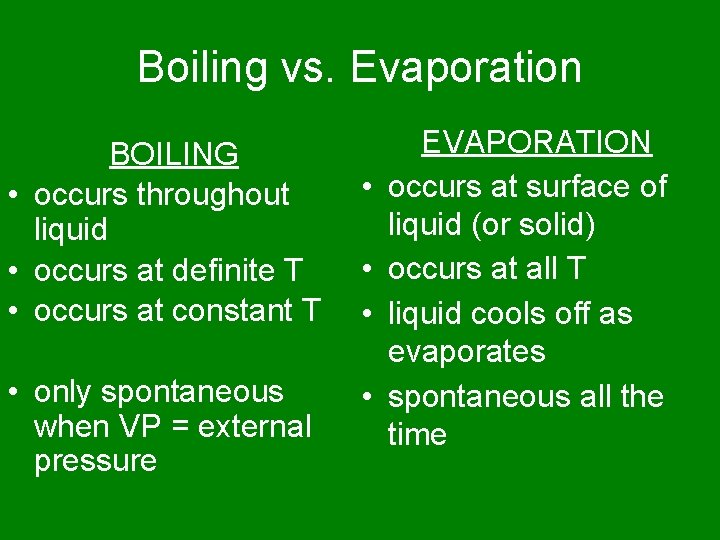
Boiling vs. Evaporation BOILING • occurs throughout liquid • occurs at definite T • occurs at constant T • only spontaneous when VP = external pressure • • EVAPORATION occurs at surface of liquid (or solid) occurs at all T liquid cools off as evaporates spontaneous all the time
Click here and go over slides from Wisc-Online before talking notes!
Vapor • Vapor is the gas phase of substance that is normally liquid at room temperature • Some evaporation occurs at all temperatures (like you saw with the ice cube) • The easier a substance evaporates, the weaker the forces between it’s molecules
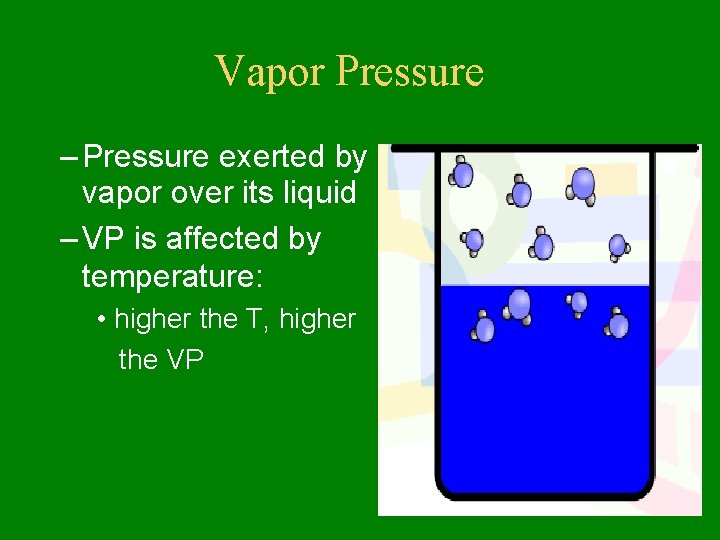
Vapor Pressure – Pressure exerted by vapor over its liquid – VP is affected by temperature: • higher the T, higher the VP

Vapor Pressure • VP does NOT depend on how much liquid is present • VP depends only on temperature
Boiling Point • Temperature at which: VP liquid = external atmospheric Pressure • Normal Boiling Point: Point temp at which VP liquid = 1 atm = standard atmospheric pressure (what we are feeling here in NY)
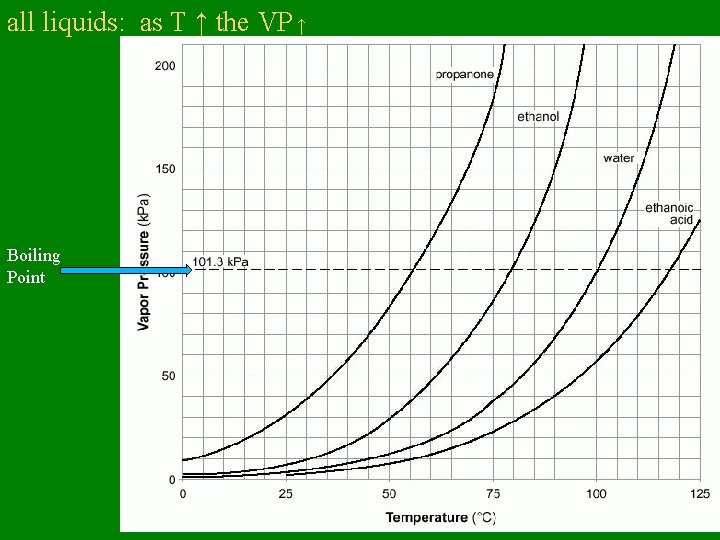
all liquids: as T ↑ the VP ↑ Boiling Point


Boiling and Pressure • If increase external pressure (ex: camping in > than 100 o. C Death Valley), boiling point is ____ • If decrease external pressure (ex: eating Raman noodles at top of Mt. Whitney), the boiling point is ____ < than 100 o. C
- Slides: 10