Topic Trends in the Periodic Table Ionization Energy

Topic: Trends in the Periodic Table: Ionization Energy and Electronegativity Do Now: p. 13 #1 -5
Trends • more than 20 properties change in predictable way based location of elements on PT • some properties: - anyone know where we can find these numbers? ! – Density – melting point/boiling point – atomic radius – ionization energy – electronegativity

REVIEW: How do you know if an atom gains or loses electrons? • Think back to the Lewis structures of ions • Atoms form ions to get a valence of 8 (or 2 for H) • Metals tend to have 1, 2, or 3 valence electrons – It’s easier to lose them • Nonmetals tend to have 5, 6, or 7 valence electrons – It’s easier to add some

OR

Ionization Energy • the amount energy required to remove a valence electron from an atom in gas phase • 1 st ionization energy = energy required to remove the most loosely held valence electron (e- farthest from nucleus) = found on table S
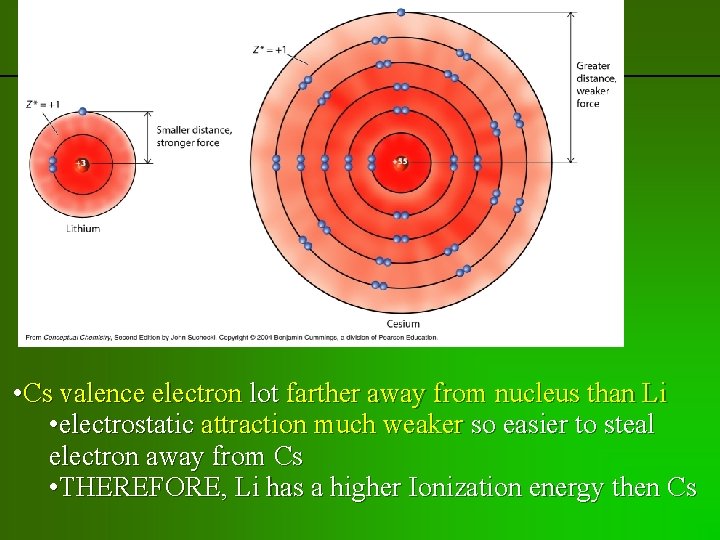
previous | index | next • Cs valence electron lot farther away from nucleus than Li • electrostatic attraction much weaker so easier to steal electron away from Cs • THEREFORE, Li has a higher Ionization energy then Cs
Increased Ionization Energy (harder to remove an electron) Decreased Ionization Energy (easier to remove an electron)
Electronegativity • ability of atom to attract electrons in bond • noble gases tend not to form bonds, so don’t have electronegativity values • Unit = Pauling • Fluorine: most electronegative element = 4. 0 Paulings
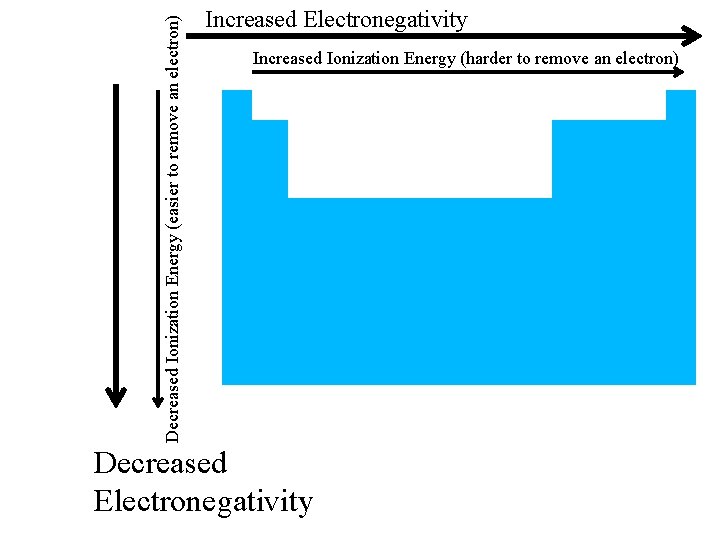
Decreased Ionization Energy (easier to remove an electron) Increased Electronegativity Increased Ionization Energy (harder to remove an electron) Decreased Electronegativity


Reactivity of Metals • judge reactivity of metals by how easily give up electrons (they’re losers) • So the easier it is to remove an electron the more reactive – Lower ionization energy = more reactive = more metallic
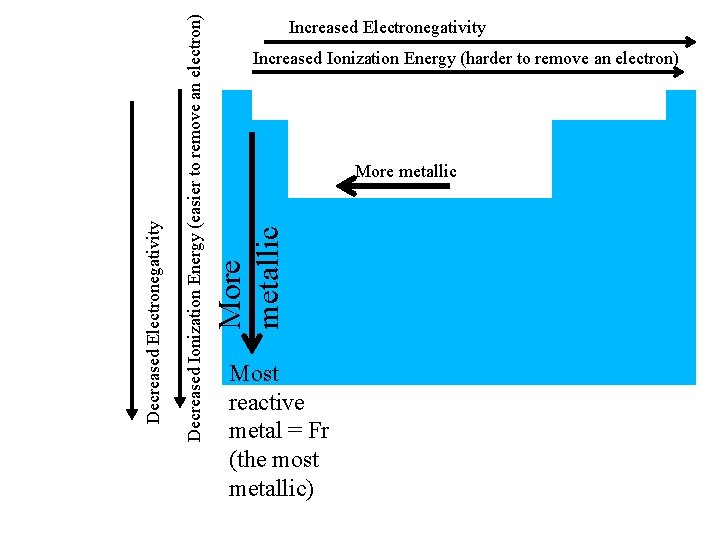
Increased Ionization Energy (harder to remove an electron) More metallic Decreased Ionization Energy (easier to remove an electron) Decreased Electronegativity Increased Electronegativity Most reactive metal = Fr (the most metallic)
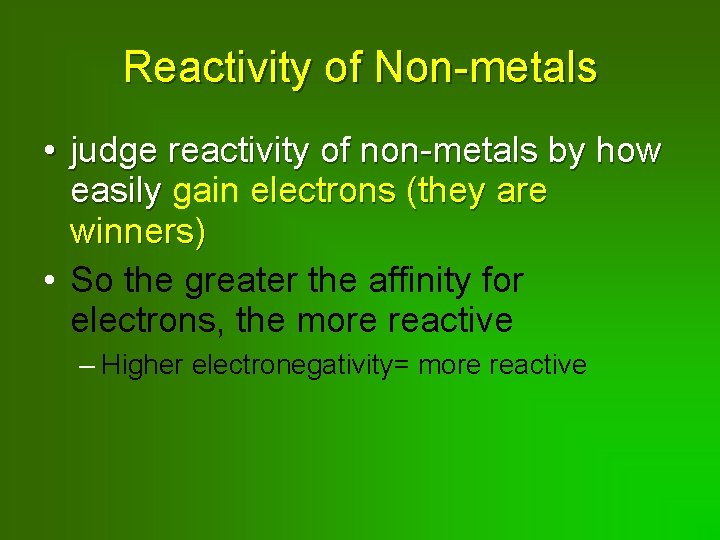
Reactivity of Non-metals • judge reactivity of non-metals by how easily gain electrons (they are winners) • So the greater the affinity for electrons, the more reactive – Higher electronegativity= more reactive
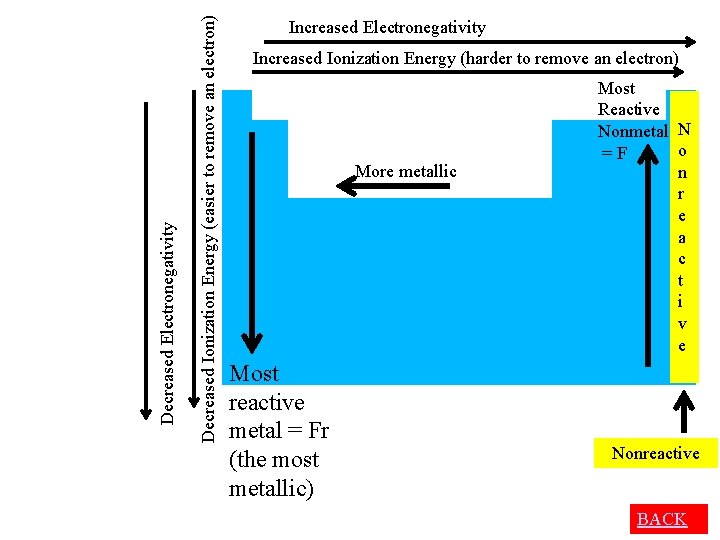
Decreased Ionization Energy (easier to remove an electron) Decreased Electronegativity Increased Ionization Energy (harder to remove an electron) More metallic Most reactive metal = Fr (the most metallic) Most Reactive Nonmetal N o =F n r e a c t i v e Nonreactive BACK

Allotropes • Different forms of element in same phase – different structures and properties • O 2 and O 3 - both gas phase – O 2 (oxygen) - necessary for life – O 3 (ozone) - toxic to life • Graphite, diamond: – both carbon in solid form
- Slides: 15