TOPIC INTRO TO CHEMISTRY CLASSIFYING MATTER Essential Question












- Slides: 22

TOPIC: INTRO TO CHEMISTRY & CLASSIFYING MATTER Essential Question: How is matter classified as an element, compound or mixture & how are solutions, suspensions and colloids identified?

1. BY THE END OF THE LESSON… YOU SHOULD BE ABLE TO … Define matter & mass and explain the difference between the two. 2. Describe the difference between Macroscopic and Microscopic levels of chemistry. 3. Explain how matter can be classified into either pure substances or mixtures. 4. Explain the difference between a heterogeneous and homogeneous mixture. 5. Explain the difference between a solution, suspension & colloid mixture.

MI#1: DIFFERENCE BETWEEN MATTER & MASS Matter= anything that has mass and takes up space Mass= a measurement reflecting the amount of matter ▪ Mass and weight are not the same ▪ Weight measures the earth’s gravitational pull on matter

MI#2: LEVELS OF CHEMISTRY Macroscopic= very large Microscopic= very small ▪ Chemistry explains how changes at a sub-microscopic level leads to changes at the macroscopic level Particles��atoms��elements��matter��everyday thin

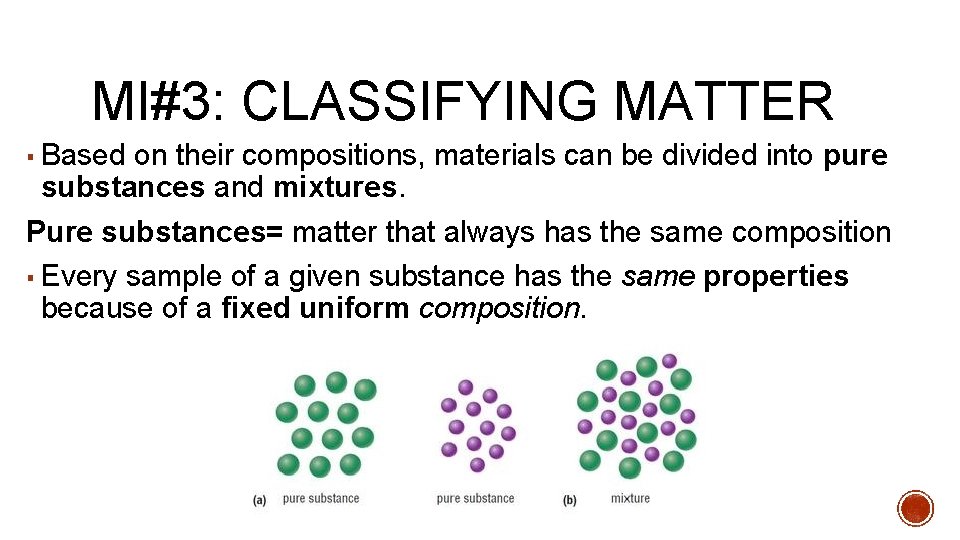
MI#3: CLASSIFYING MATTER ▪ Based on their compositions, materials can be divided into pure substances and mixtures. Pure substances= matter that always has the same composition ▪ Every sample of a given substance has the same properties because of a fixed uniform composition.

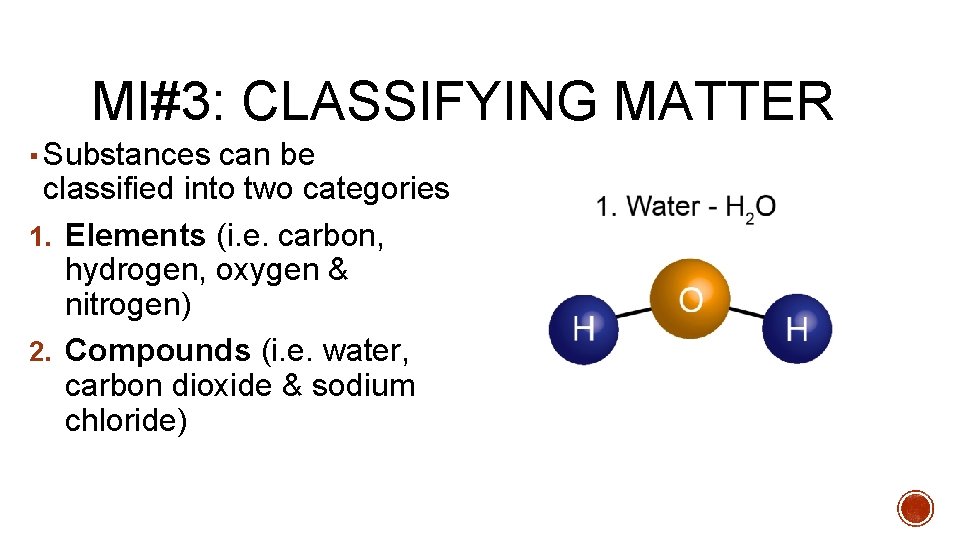
MI#3: CLASSIFYING MATTER ▪ Substances can be classified into two categories 1. Elements (i. e. carbon, hydrogen, oxygen & nitrogen) 2. Compounds (i. e. water, carbon dioxide & sodium chloride)

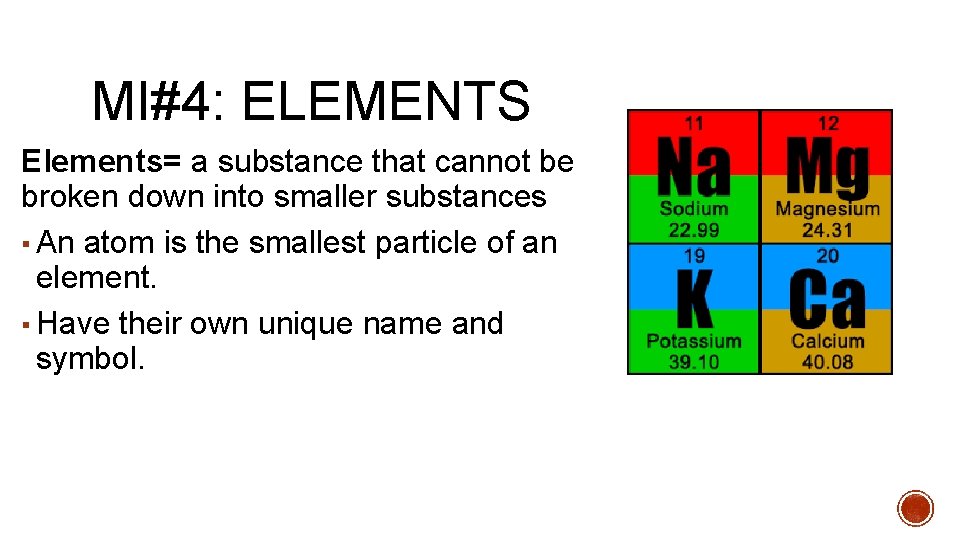
MI#4: ELEMENTS Elements= a substance that cannot be broken down into smaller substances ▪ An atom is the smallest particle of an element. ▪ Have their own unique name and symbol.

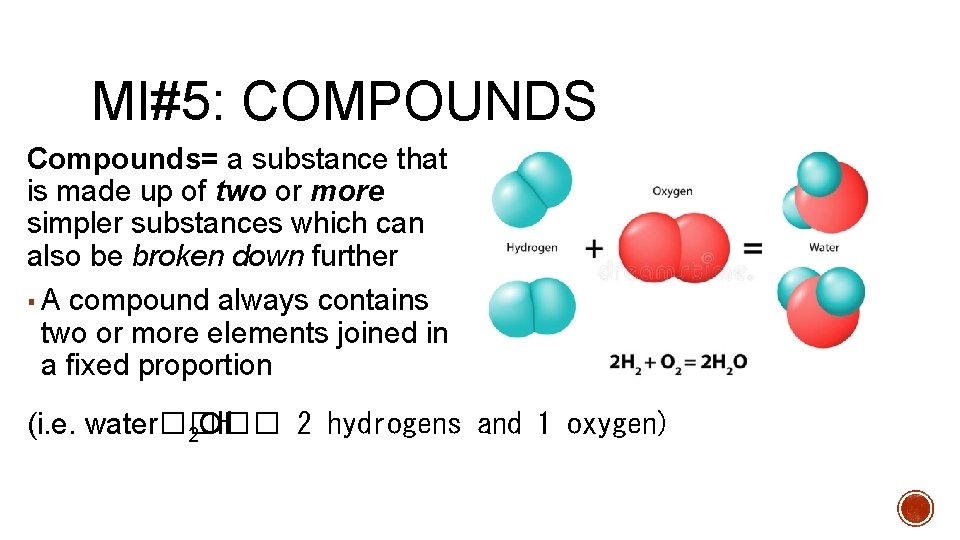
MI#5: COMPOUNDS Compounds= a substance that is made up of two or more simpler substances which can also be broken down further ▪ A compound always contains two or more elements joined in a fixed proportion (i. e. water��H 2 O�� 2 hydrogens and 1 oxygen)

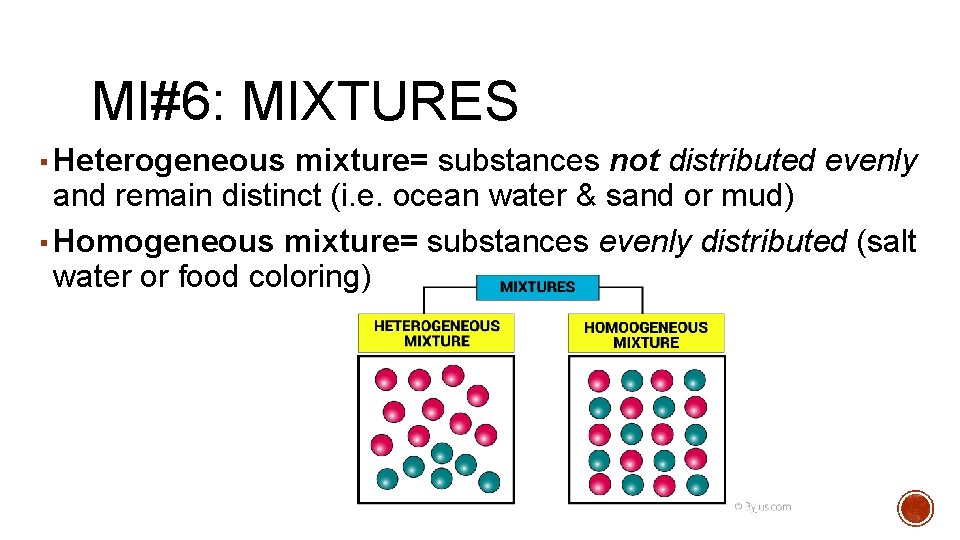
MI#6: MIXTURES Mixtures= a combination of two or more pure substances and each pure substance retains its individual properties

MI#6: MIXTURES ▪ Heterogeneous mixture= substances not distributed evenly and remain distinct (i. e. ocean water & sand or mud) ▪ Homogeneous mixture= substances evenly distributed (salt water or food coloring)

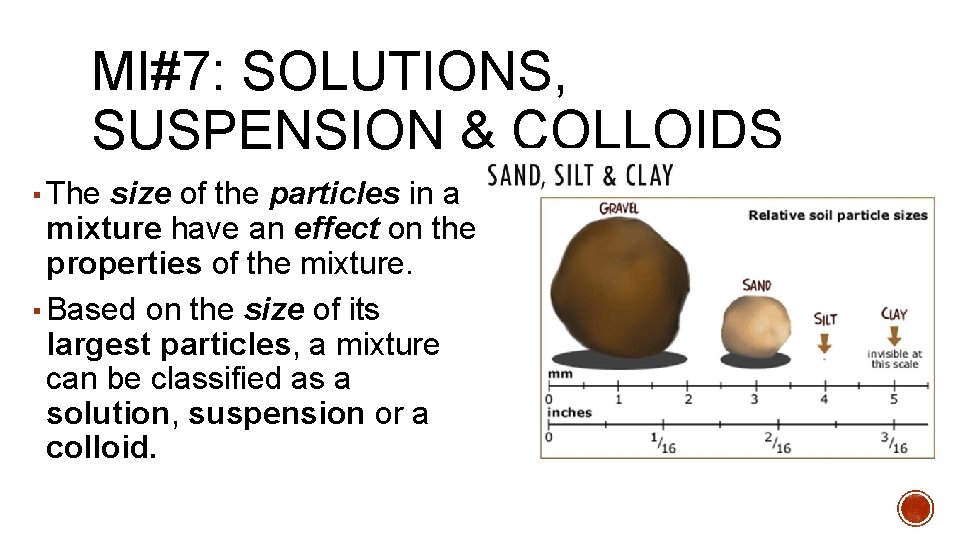
MI#7: SOLUTIONS, SUSPENSION & COLLOIDS ▪ The size of the particles in a mixture have an effect on the properties of the mixture. ▪ Based on the size of its largest particles, a mixture can be classified as a solution, suspension or a colloid.

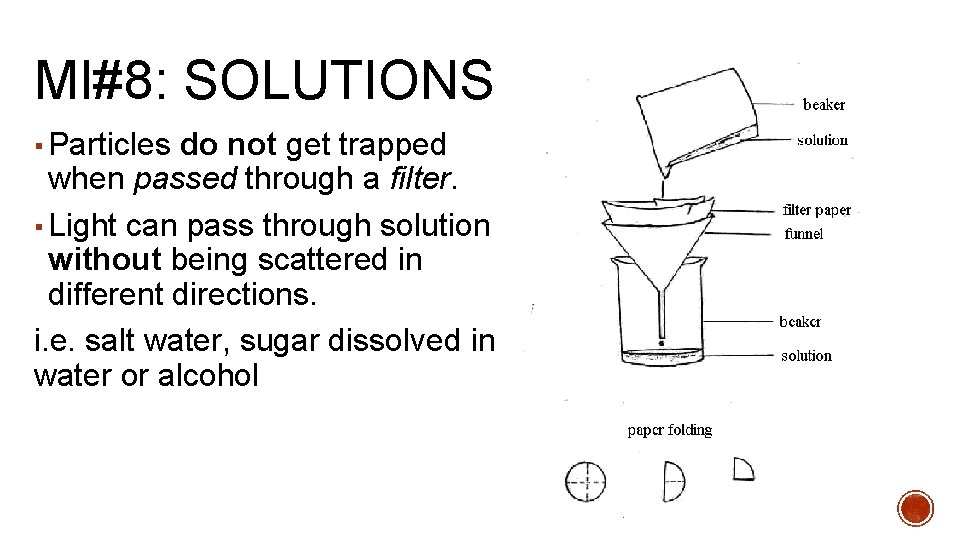
MI#8: SOLUTIONS Solutions= formed when substances dissolve and form a homogeneous mixture ▪ Do not separate into distinct layers. ▪ Particles too small to settle out of the solution.


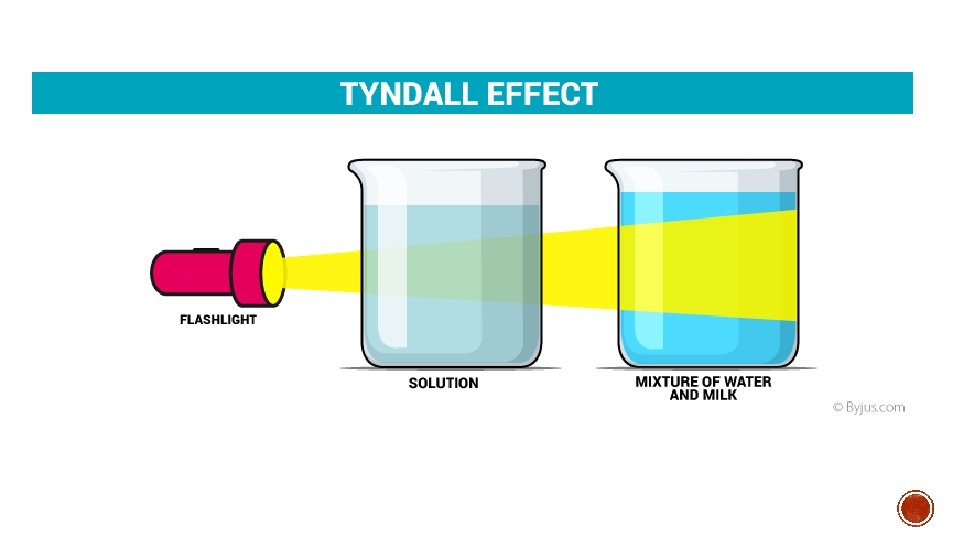
MI#8: SOLUTIONS ▪ Particles do not get trapped when passed through a filter. ▪ Light can pass through solution without being scattered in different directions. i. e. salt water, sugar dissolved in water or alcohol


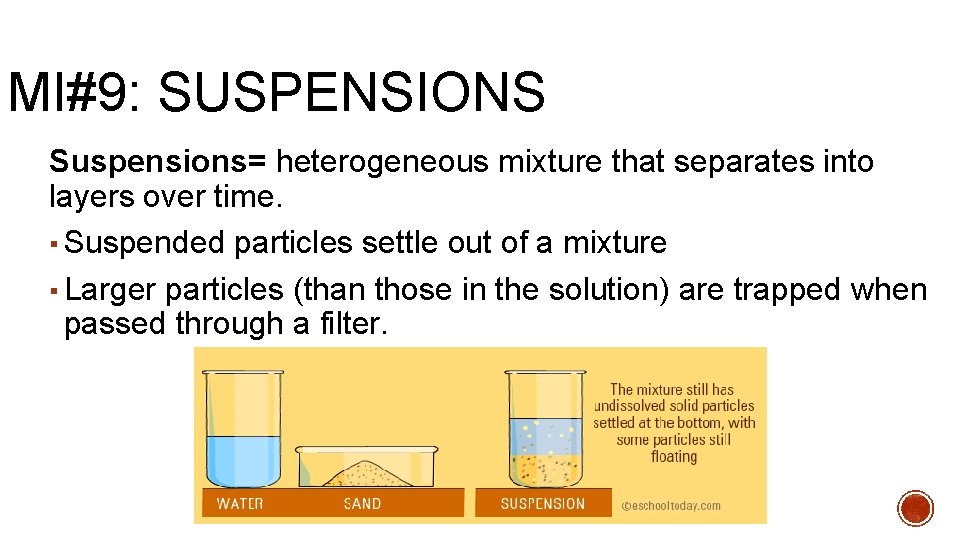
MI#9: SUSPENSIONS Suspensions= heterogeneous mixture that separates into layers over time. ▪ Suspended particles settle out of a mixture ▪ Larger particles (than those in the solution) are trapped when passed through a filter.

MI#9: SUSPENSIONS ▪ Suspensions are cloudy due to larger particles and scatter light. i. e. muddy water or sand with water

MI#10: COLLOIDS Colloids= contains particles that are intermediate in size ▪ Do not separate into distinct layers. ▪ Particles too small to settle out of the solution.

MI#10: COLLOIDS ▪ Particles do not get trapped when passed through a filter. ▪ Light is scattered i. e. milk, butter, or smoke (a solid dispersed in a gas)

GUESS THE MIXTURE…

WHERE ARE YOU AT NOW? CAN YOU SAY YOU ARE ABLE TO… 1. Define matter & mass and explain the difference between the two. 2. Describe the difference between Macroscopic and Microscopic levels of chemistry. 3. Explain how matter can be classified into either pure substances or mixtures. 4. Explain the difference between a heterogeneous and homogeneous mixture. 5. Explain the difference between a solution, suspension & colloid mixture.

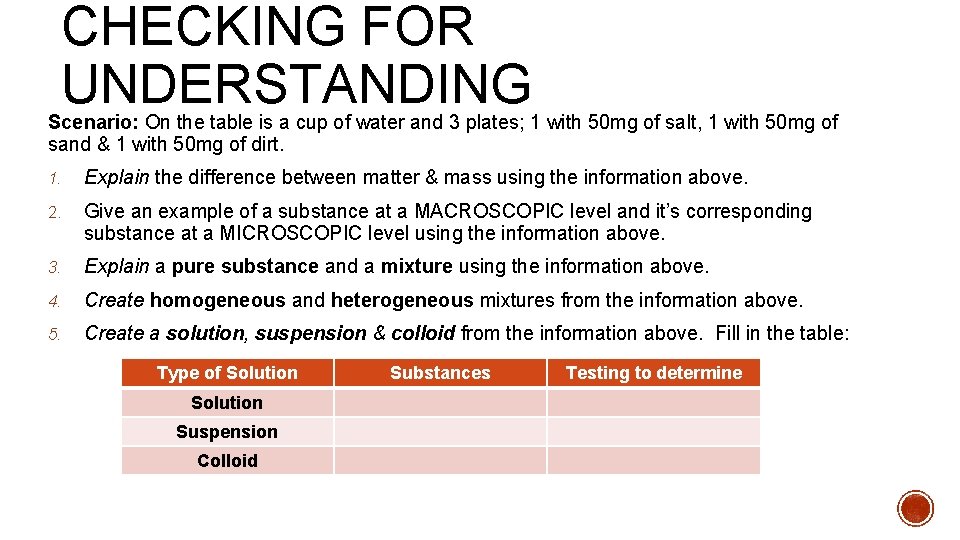
CHECKING FOR UNDERSTANDING Scenario: On the table is a cup of water and 3 plates; 1 with 50 mg of salt, 1 with 50 mg of sand & 1 with 50 mg of dirt. 1. Explain the difference between matter & mass using the information above. 2. Give an example of a substance at a MACROSCOPIC level and it’s corresponding substance at a MICROSCOPIC level using the information above. 3. Explain a pure substance and a mixture using the information above. 4. Create homogeneous and heterogeneous mixtures from the information above. 5. Create a solution, suspension & colloid from the information above. Fill in the table: Type of Solution Suspension Colloid Substances Testing to determine