Topic 8 Energetics 1 Standard enthalpy of Combustion

Topic 8 Energetics 1 Standard enthalpy of Combustion
Enthalpy Changes of Combustion • be able to calculate enthalpy changes in k. J mol-1 from given experimental results Both a sign and units are expected in the final answer. • understand experiments to measure enthalpy changes in terms of: i processing results using the expression: energy transferred = mass x specific heat capacity × temperature change (Q=mcΔT) ii evaluating sources of error and assumptions made in the experiments • Students will need to consider experiments where: • enthalpy of combustion is measured, such as using a series of alcohols in a spirit burner

Definitions • Without referring to your notes, write a definition of: Enthalpy change of combustion The enthalpy change that takes place when one mole of a substance reacts completely with oxygen under standard conditions, all reactants and products in their standard states.
Standard Enthalpy Change of combustion Standard enthalpy change of combustion, ΔHcӨ Close your text books Write equations for the standard enthalpy of combustion of: • CH 4 ΔHcӨ = -890 • H 2 ΔHcӨ = -286 • Al ΔHcӨ = -1676 Write out the equations in full including state symbols, and the appropriate units.

Experimental determination of standard enthalpy change of combustion What do you need to do to measure a ΔHcӨ? 1. Burn a known mass of fuel 2. Heat a known mass of water 3. Measure the temperature change of the water On the paper, draw apparatus to do this.
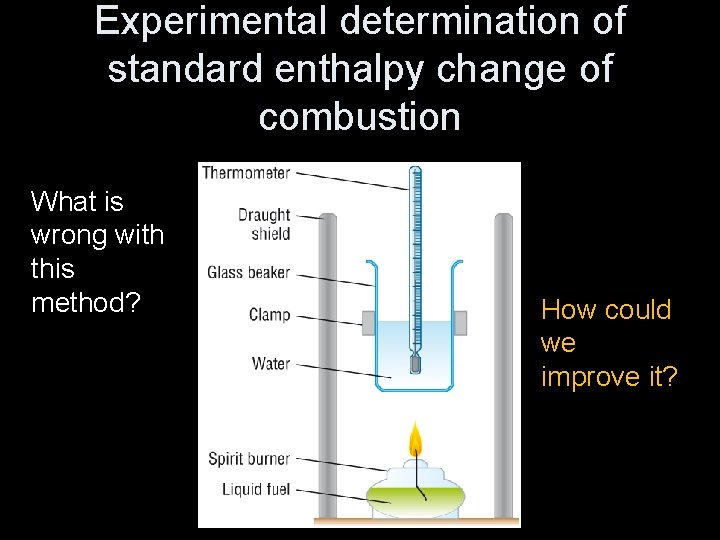
Experimental determination of standard enthalpy change of combustion What is wrong with this method? How could we improve it?

Practice… • • 2 g of propan-1 -ol heated 250 cm 3 of water by 50°C. Find the enthalpy change of combustion of propan-1 -ol Specific heat capacity water =4. 18 J. g-1. K-1 Density of water = 1 g. cm-3 Help: 1. 2. 3. 4. Find heat change of experiment Find the amount in moles of propanol that reacted Work out heat loss in k. J. mol-1 Report answer ΔHcӨ

Experimental determination of standard enthalpy change of combustion In these experiments we often end up with a value for ΔHcӨ that is too low. Why? 1. There may have been incomplete combustion 2. There may have been heat loss to the surroundings (not heating the water) How could we reduce these?
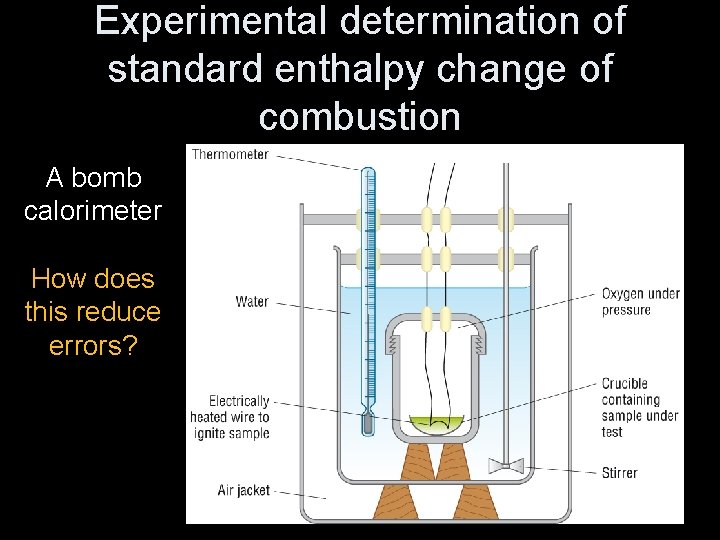
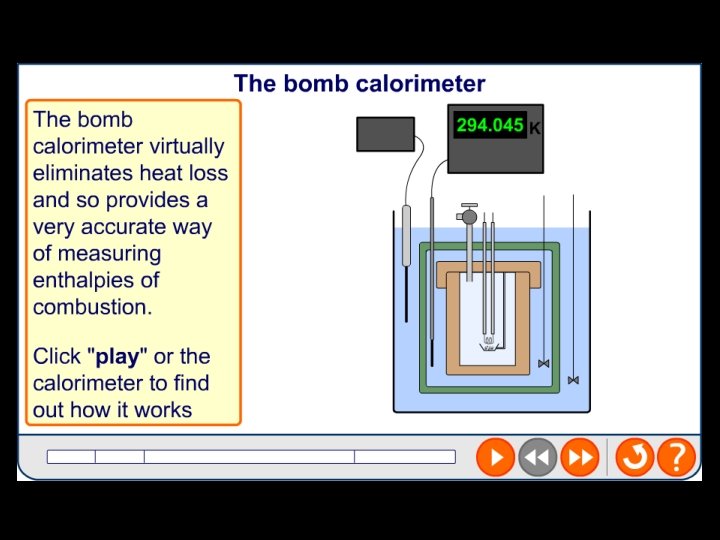
Experimental determination of standard enthalpy change of combustion A bomb calorimeter How does this reduce errors?

• Past exam questions

1) Calculate the enthalpy changes of combustion for butan-1 -ol and decane using the experimental results below. Give both answers to three significant figures. a) 1. 51 g of butan – 1 – ol, CH 3 CH 2 CH 2 OH, heated 300 cm 3 of water by 42. 0 o. C. b) 0. 826 g of decane heated 180 cm 3 of water from 22. 0 o. C to 71. 0 o. C
Enthalpy Changes of Combustion • be able to calculate enthalpy changes in k. J mol-1 from given experimental results Both a sign and units are expected in the final answer. • understand experiments to measure enthalpy changes in terms of: i processing results using the expression: energy transferred = mass x specific heat capacity × temperature change (Q=mcΔT) ii evaluating sources of error and assumptions made in the experiments • Students will need to consider experiments where: • enthalpy of combustion is measured, such as using a series of alcohols in a spirit burner
- Slides: 14