Topic 7 T Cell Development Repertoire Selection and






- Slides: 77

Topic 7 T Cell Development, Repertoire Selection and Immune Self Tolerance ©Dr. Colin R. A. Hewitt crah 1@le. ac. uk

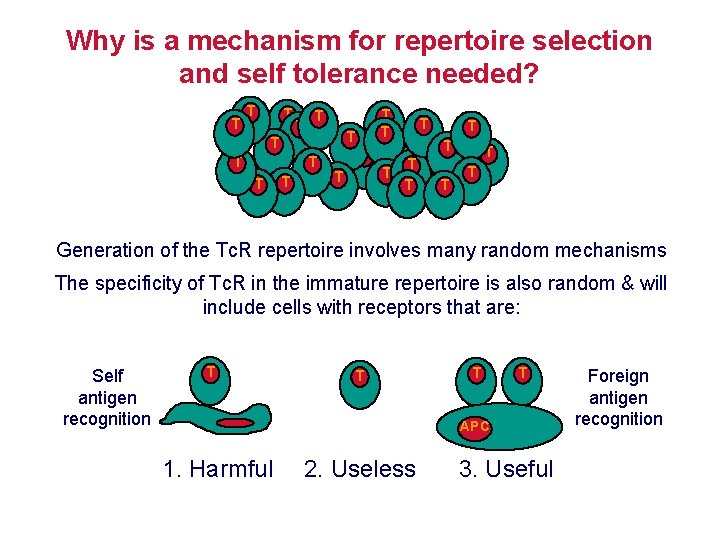
Why is a mechanism for repertoire selection and self tolerance needed? T T T T T T T Generation of the Tc. R repertoire involves many random mechanisms The specificity of Tc. R in the immature repertoire is also random & will include cells with receptors that are: Self antigen recognition T T APC 1. Harmful 2. Useless 3. Useful Foreign antigen recognition

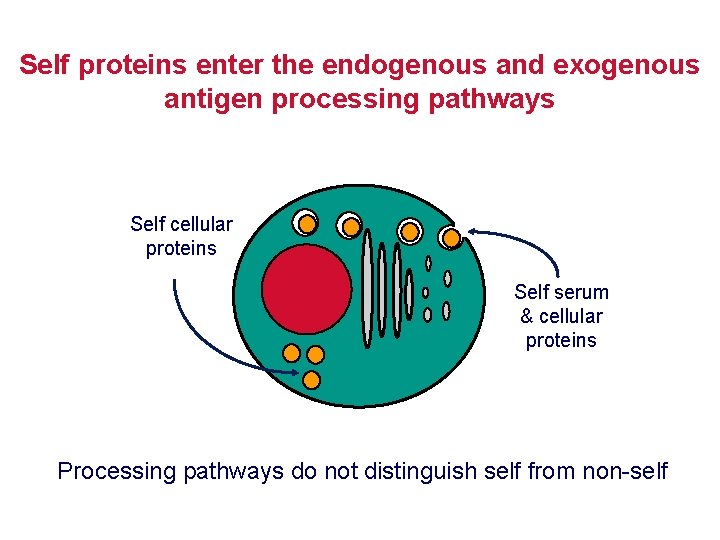
Self proteins enter the endogenous and exogenous antigen processing pathways Self cellular proteins Self serum & cellular proteins Processing pathways do not distinguish self from non-self

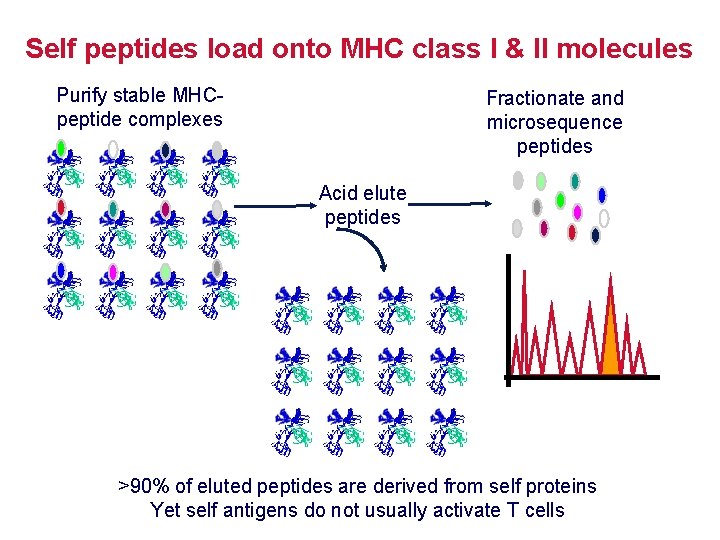
Self peptides load onto MHC class I & II molecules Purify stable MHCpeptide complexes Fractionate and microsequence peptides Acid elute peptides >90% of eluted peptides are derived from self proteins Yet self antigens do not usually activate T cells

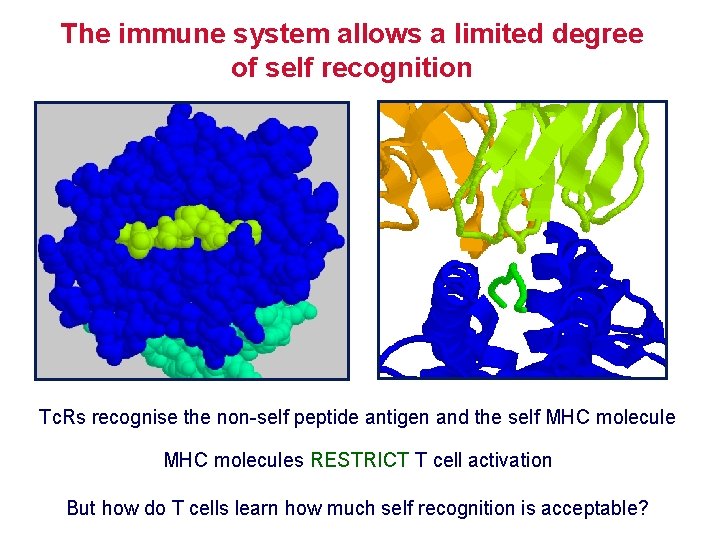
The immune system allows a limited degree of self recognition Tc. Rs recognise the non-self peptide antigen and the self MHC molecules RESTRICT T cell activation But how do T cells learn how much self recognition is acceptable?

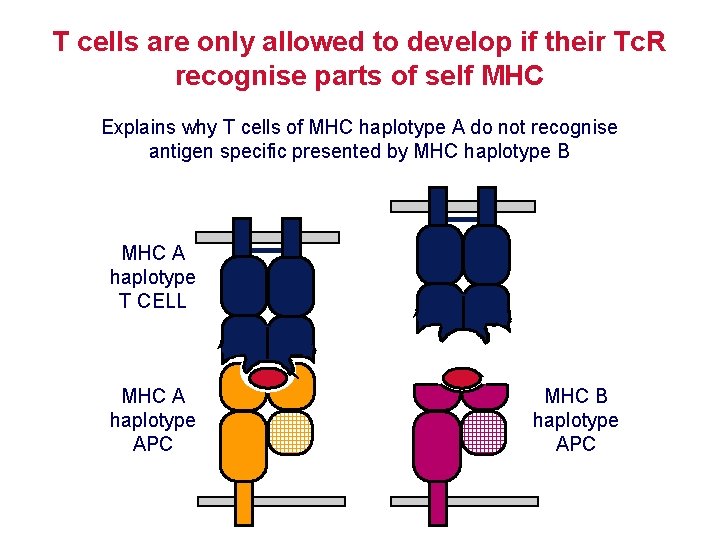
T cells are only allowed to develop if their Tc. R recognise parts of self MHC Explains why T cells of MHC haplotype A do not recognise antigen specific presented by MHC haplotype B MHC A haplotype T CELL MHC A haplotype APC MHC B haplotype APC

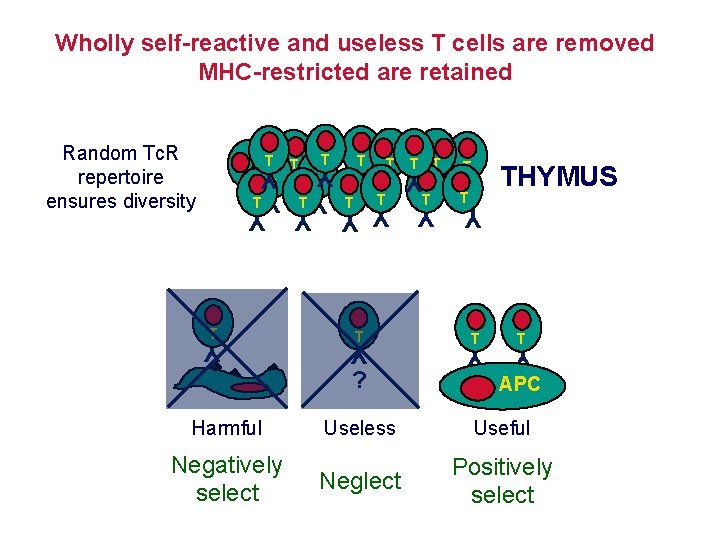
Wholly self-reactive and useless T cells are removed MHC-restricted are retained T T T T Y Y YYYYY Y YY T T T Y ? Harmful Negatively select T T T Y T THYMUS T Y Random Tc. R repertoire ensures diversity APC Useless Useful Neglect Positively select

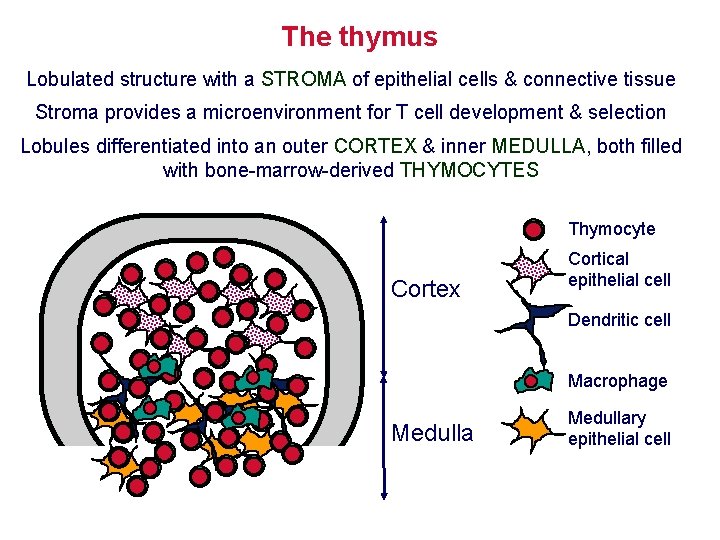
The thymus Lobulated structure with a STROMA of epithelial cells & connective tissue Stroma provides a microenvironment for T cell development & selection Lobules differentiated into an outer CORTEX & inner MEDULLA, both filled with bone-marrow-derived THYMOCYTES Thymocyte Cortex Cortical epithelial cell Dendritic cell Macrophage Medullary epithelial cell

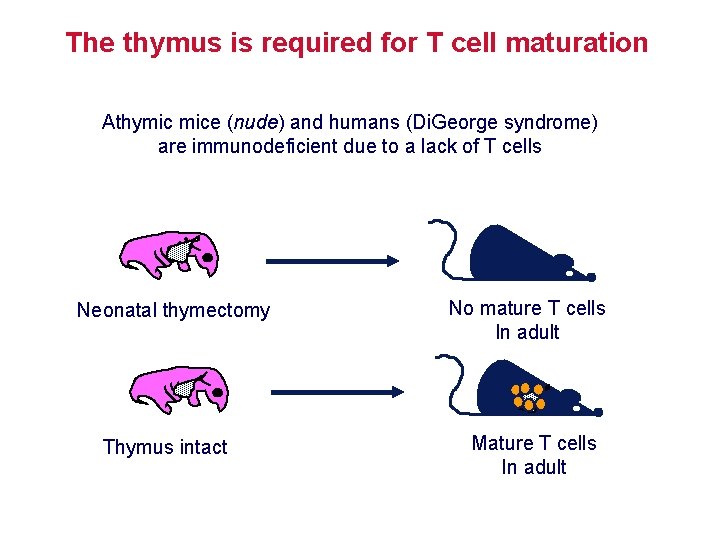
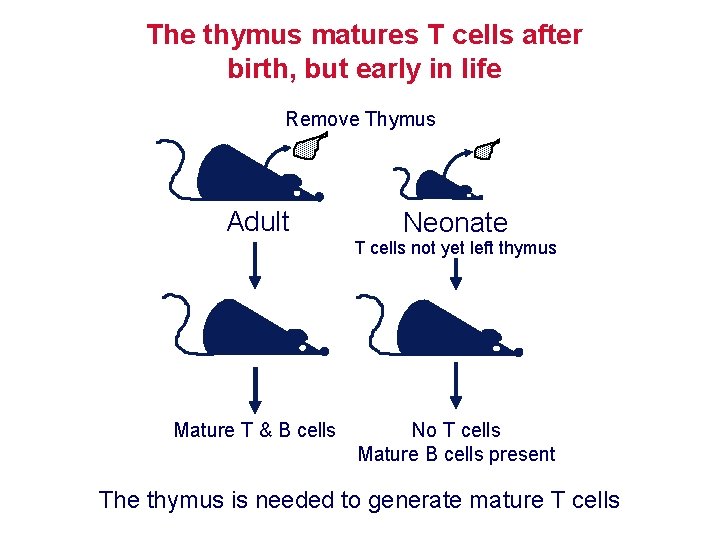
The thymus is required for T cell maturation Athymic mice (nude) and humans (Di. George syndrome) are immunodeficient due to a lack of T cells Neonatal thymectomy Thymus intact No mature T cells In adult Mature T cells In adult

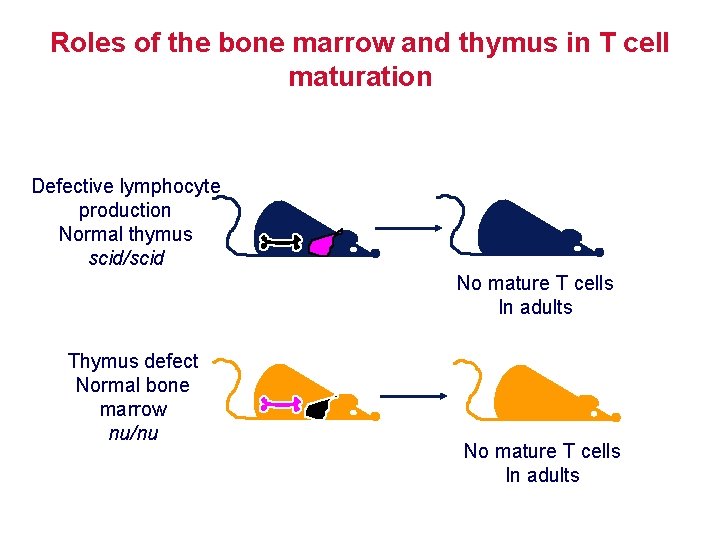
Roles of the bone marrow and thymus in T cell maturation Defective lymphocyte production Normal thymus scid/scid No mature T cells In adults Thymus defect Normal bone marrow nu/nu No mature T cells In adults

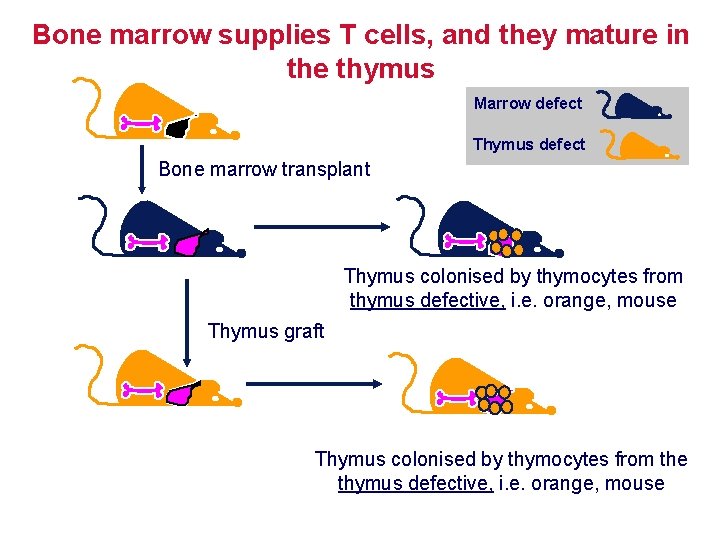
Bone marrow supplies T cells, and they mature in the thymus Marrow defect Thymus defect Bone marrow transplant Thymus colonised by thymocytes from thymus defective, i. e. orange, mouse Thymus graft Thymus colonised by thymocytes from the thymus defective, i. e. orange, mouse

The thymus matures T cells after birth, but early in life Remove Thymus Adult Neonate T cells not yet left thymus Mature T & B cells No T cells Mature B cells present The thymus is needed to generate mature T cells

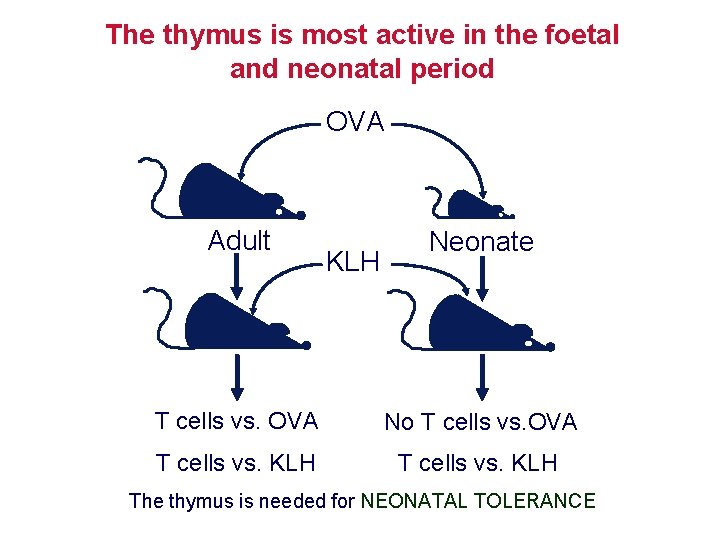
The thymus is most active in the foetal and neonatal period OVA Adult KLH Neonate T cells vs. OVA No T cells vs. OVA T cells vs. KLH The thymus is needed for NEONATAL TOLERANCE

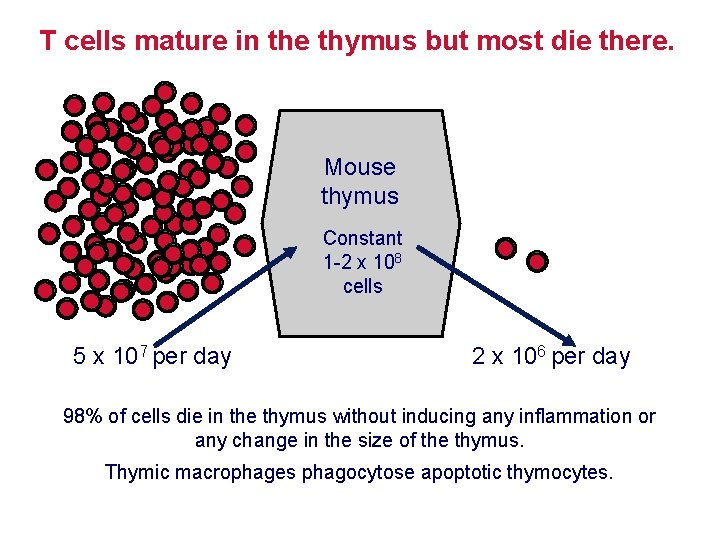
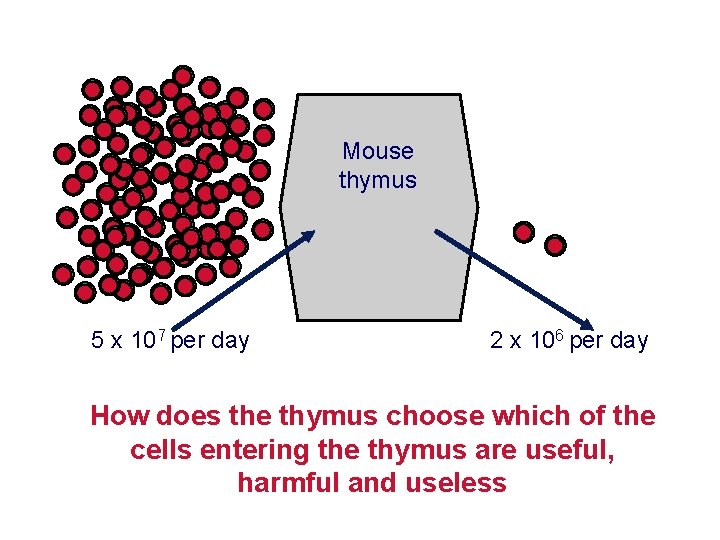
T cells mature in the thymus but most die there. Mouse thymus Constant 1 -2 x 108 cells 5 x 107 per day 2 x 106 per day 98% of cells die in the thymus without inducing any inflammation or any change in the size of the thymus. Thymic macrophages phagocytose apoptotic thymocytes.

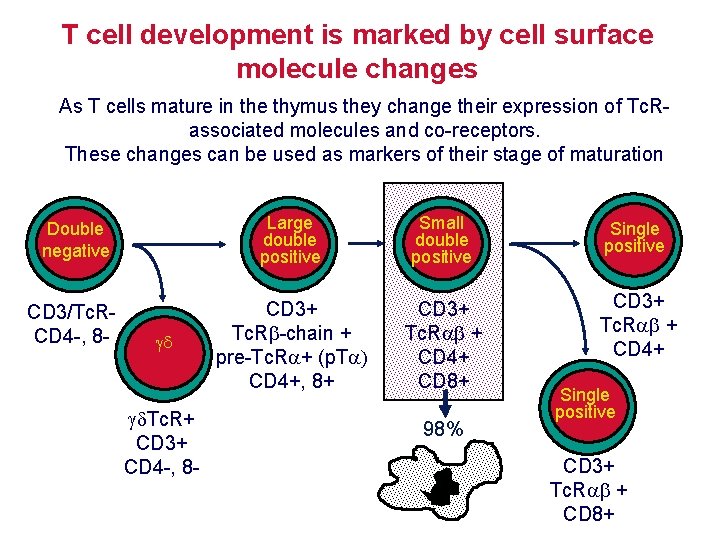
T cell development is marked by cell surface molecule changes As T cells mature in the thymus they change their expression of Tc. Rassociated molecules and co-receptors. These changes can be used as markers of their stage of maturation Double negative Large double positive Small double positive Single positive CD 3/Tc. RCD 4 -, 8 - CD 3+ Tc. R -chain + pre-Tc. R + (p. T CD 4+, 8+ CD 3+ Tc. R + CD 4+ CD 8+ CD 3+ Tc. R + CD 4+ Tc. R+ CD 3+ CD 4 -, 8 - 98% Single positive CD 3+ Tc. R + CD 8+

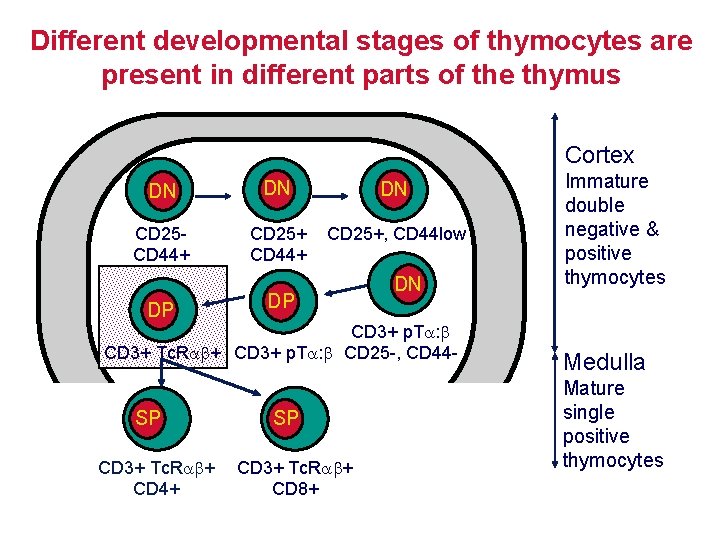
Different developmental stages of thymocytes are present in different parts of the thymus Cortex DN DN DN CD 25 CD 44+ CD 25+, CD 44 low DP DP DN CD 3+ p. T : CD 3+ Tc. R + CD 3+ p. T : CD 25 -, CD 44 - SP CD 3+ Tc. R + CD 4+ SP CD 3+ Tc. R + CD 8+ Immature double negative & positive thymocytes Medulla Mature single positive thymocytes

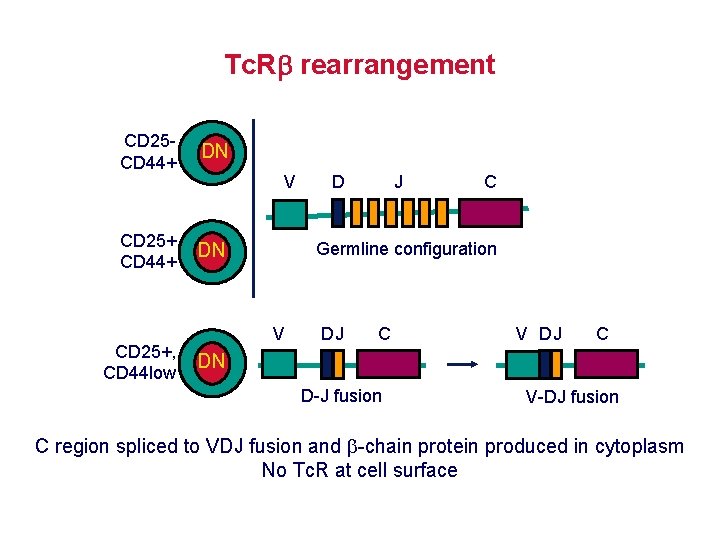
Tc. R rearrangement CD 25 CD 44+ DN CD 25+, CD 44 low V D J C Germline configuration V DJ C DN D-J fusion V-DJ fusion C region spliced to VDJ fusion and -chain protein produced in cytoplasm No Tc. R at cell surface

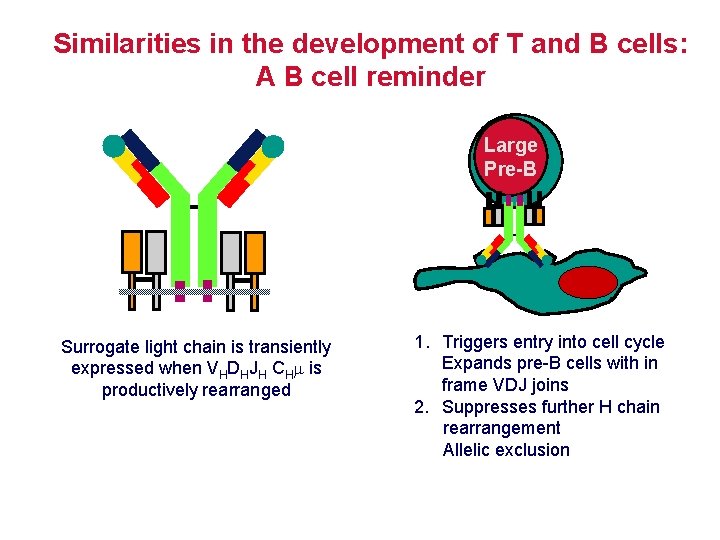
Similarities in the development of T and B cells: A B cell reminder Large Pre-B Surrogate light chain is transiently expressed when VHDHJH CHm is productively rearranged 1. Triggers entry into cell cycle Expands pre-B cells with in frame VDJ joins 2. Suppresses further H chain rearrangement Allelic exclusion

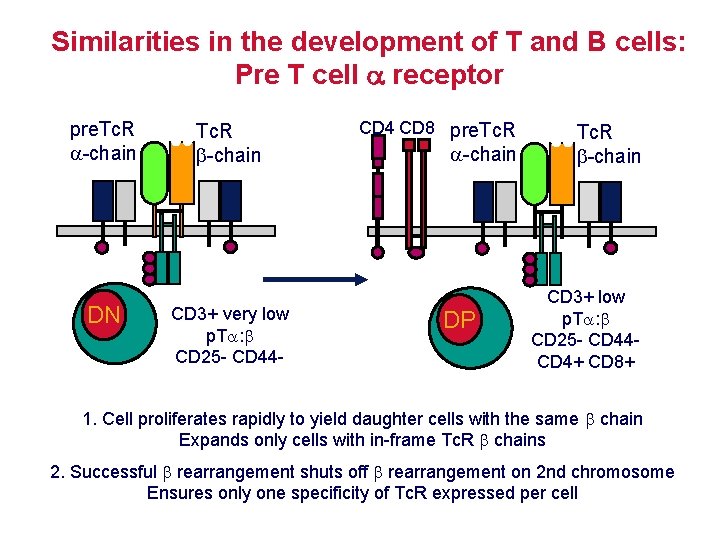
Similarities in the development of T and B cells: Pre T cell receptor pre. Tc. R -chain DN Tc. R -chain CD 3+ very low p. T : CD 25 - CD 44 - CD 4 CD 8 pre. Tc. R -chain DP Tc. R -chain CD 3+ low p. T : CD 25 - CD 44 CD 4+ CD 8+ 1. Cell proliferates rapidly to yield daughter cells with the same chain Expands only cells with in-frame Tc. R chains 2. Successful rearrangement shuts off rearrangement on 2 nd chromosome Ensures only one specificity of Tc. R expressed per cell

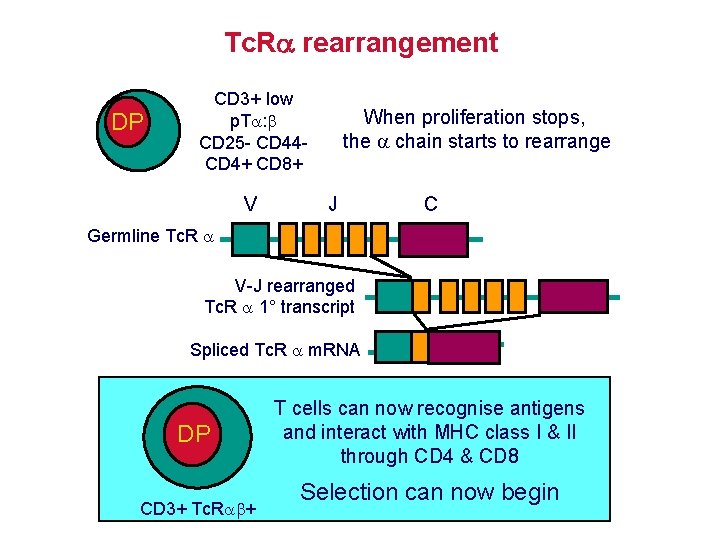
Tc. R rearrangement DP CD 3+ low p. T : CD 25 - CD 44 CD 4+ CD 8+ V When proliferation stops, the chain starts to rearrange J C Germline Tc. R V-J rearranged Tc. R 1° transcript Spliced Tc. R m. RNA DP CD 3+ Tc. R + T cells can now recognise antigens and interact with MHC class I & II through CD 4 & CD 8 Selection can now begin

Mouse thymus 5 x 107 per day 2 x 106 per day How does the thymus choose which of the cells entering the thymus are useful, harmful and useless

Sorting the useful from the harmful and the useless Positive selection Retention of thymocytes expressing Tc. R that are RESTRICTED in their recognition of antigen by self MHC i. e. selection of the USEFUL Negative selection Removal of thymocytes expressing Tc. R that either recognise self antigens presented by self MHC or that have no affinity for self MHC i. e. selection of the HARMFUL and the USELESS

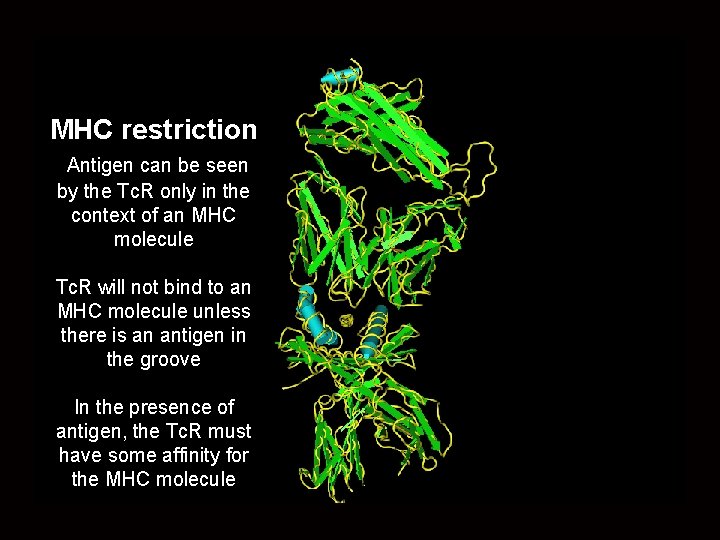
MHC restriction Antigen can be seen by the Tc. R only in the context of an MHC molecule Tc. R will not bind to an MHC molecule unless there is an antigen in the groove In the presence of antigen, the Tc. R must have some affinity for the MHC molecule

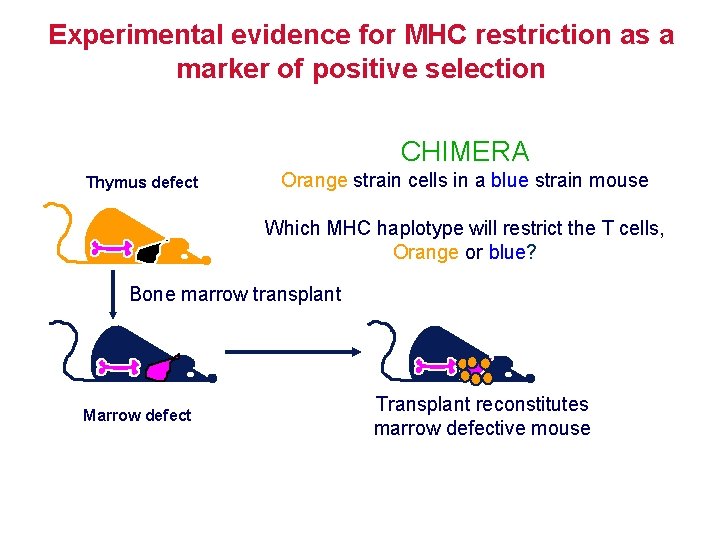
Experimental evidence for MHC restriction as a marker of positive selection CHIMERA Thymus defect Orange strain cells in a blue strain mouse Which MHC haplotype will restrict the T cells, Orange or blue? Bone marrow transplant Marrow defect Transplant reconstitutes marrow defective mouse

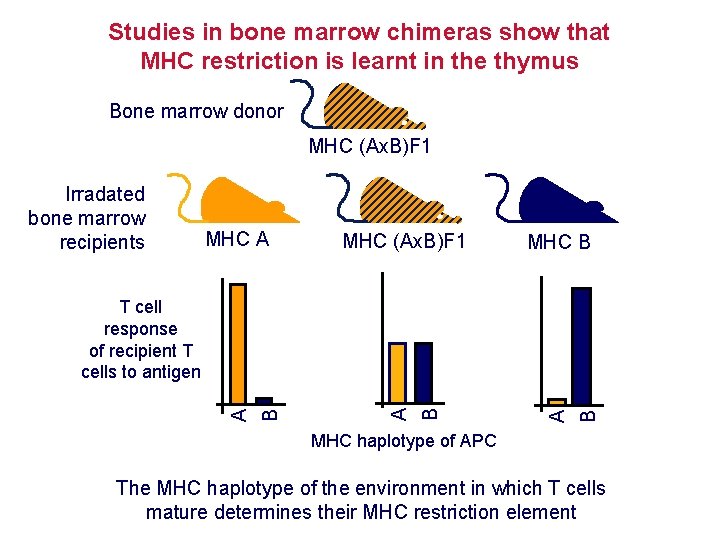
Studies in bone marrow chimeras show that MHC restriction is learnt in the thymus Bone marrow donor MHC A MHC (Ax. B)F 1 MHC B A Irradated bone marrow recipients A MHC (Ax. B)F 1 B B B A T cell response of recipient T cells to antigen MHC haplotype of APC The MHC haplotype of the environment in which T cells mature determines their MHC restriction element

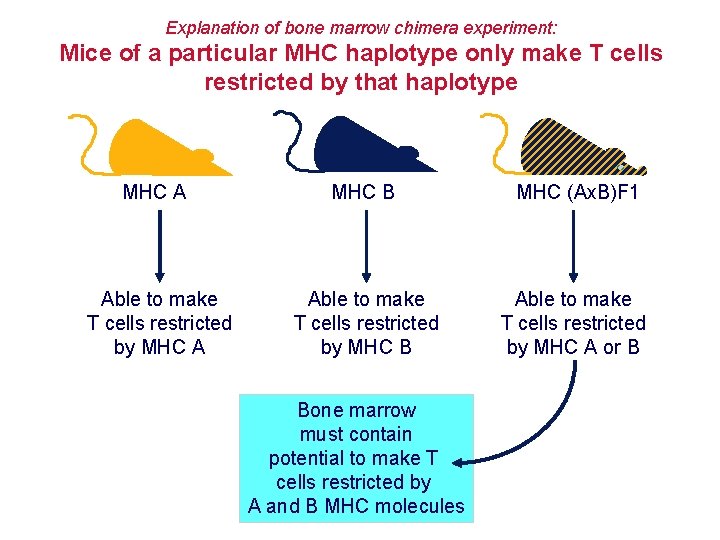
Explanation of bone marrow chimera experiment: Mice of a particular MHC haplotype only make T cells restricted by that haplotype MHC A MHC B MHC (Ax. B)F 1 Able to make T cells restricted by MHC A Able to make T cells restricted by MHC B Able to make T cells restricted by MHC A or B Bone marrow must contain potential to make T cells restricted by A and B MHC molecules

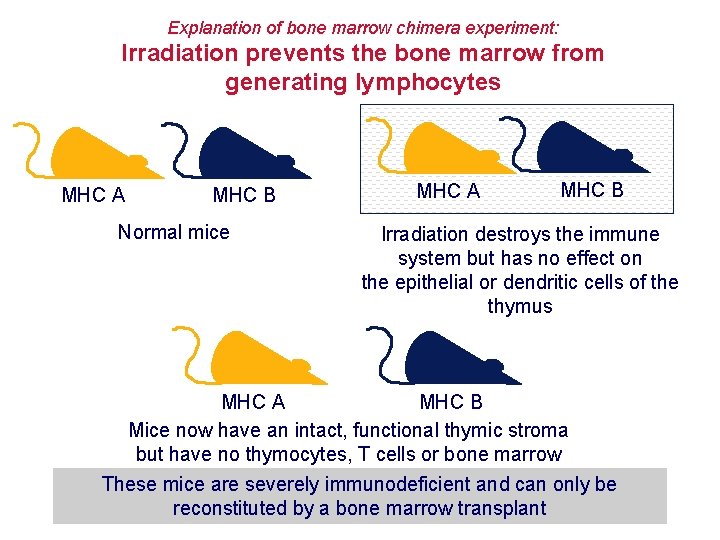
Explanation of bone marrow chimera experiment: Irradiation prevents the bone marrow from generating lymphocytes MHC A MHC B Normal mice MHC A MHC B Irradiation destroys the immune system but has no effect on the epithelial or dendritic cells of the thymus MHC A MHC B Mice now have an intact, functional thymic stroma but have no thymocytes, T cells or bone marrow These mice are severely immunodeficient and can only be reconstituted by a bone marrow transplant

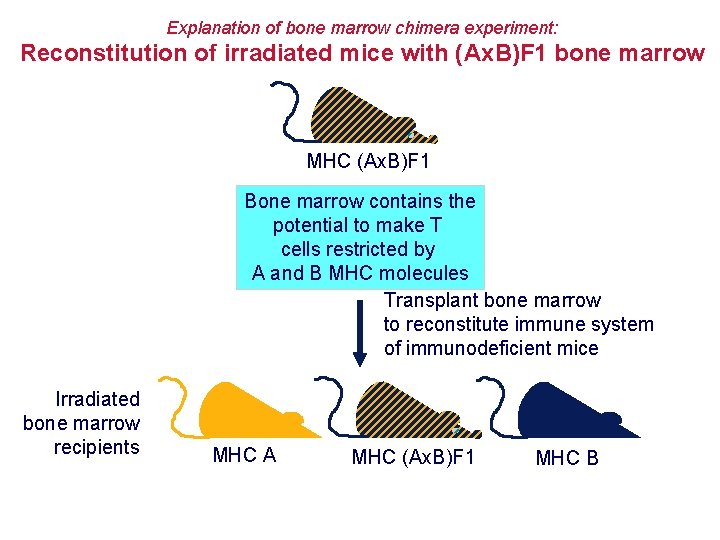
Explanation of bone marrow chimera experiment: Reconstitution of irradiated mice with (Ax. B)F 1 bone marrow MHC (Ax. B)F 1 Bone marrow contains the potential to make T cells restricted by A and B MHC molecules Transplant bone marrow to reconstitute immune system of immunodeficient mice Irradiated bone marrow recipients MHC A MHC (Ax. B)F 1 MHC B

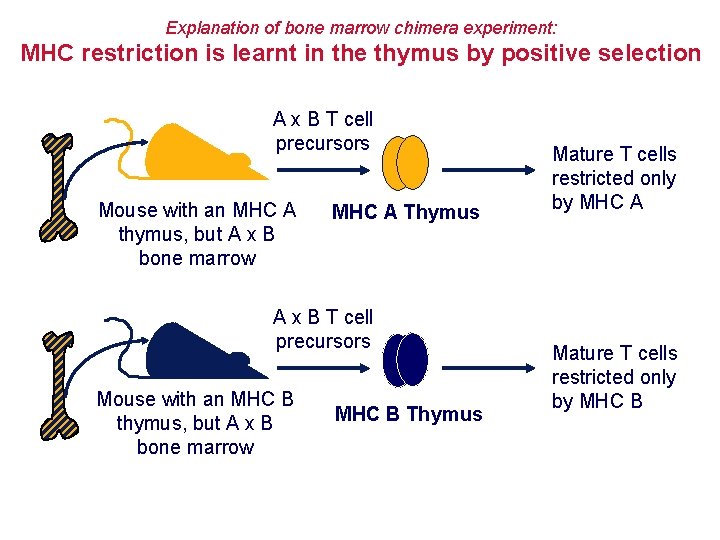
Explanation of bone marrow chimera experiment: MHC restriction is learnt in the thymus by positive selection A x B T cell precursors Mouse with an MHC A thymus, but A x B bone marrow MHC A Thymus A x B T cell precursors Mouse with an MHC B thymus, but A x B bone marrow MHC B Thymus Mature T cells restricted only by MHC A Mature T cells restricted only by MHC B

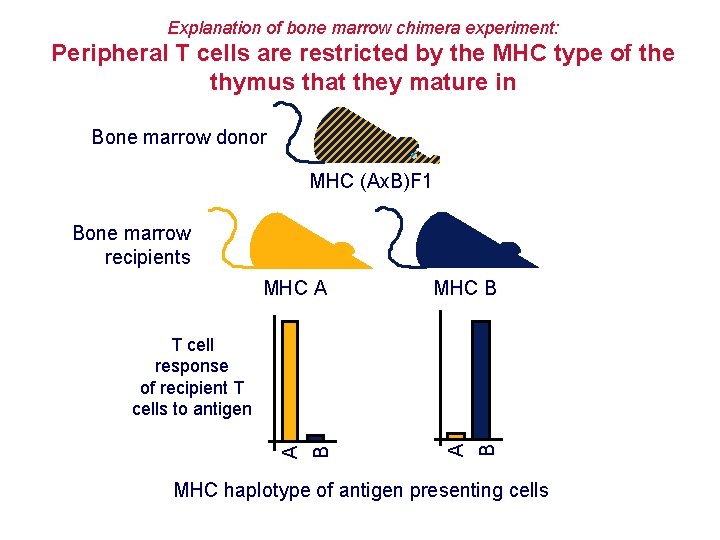
Explanation of bone marrow chimera experiment: Peripheral T cells are restricted by the MHC type of the thymus that they mature in Bone marrow donor MHC (Ax. B)F 1 Bone marrow recipients MHC A MHC B B A T cell response of recipient T cells to antigen MHC haplotype of antigen presenting cells

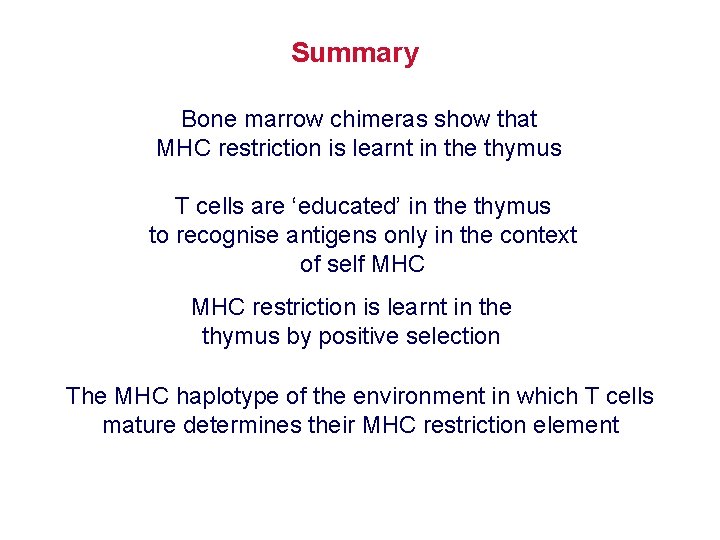
Summary Bone marrow chimeras show that MHC restriction is learnt in the thymus T cells are ‘educated’ in the thymus to recognise antigens only in the context of self MHC restriction is learnt in the thymus by positive selection The MHC haplotype of the environment in which T cells mature determines their MHC restriction element

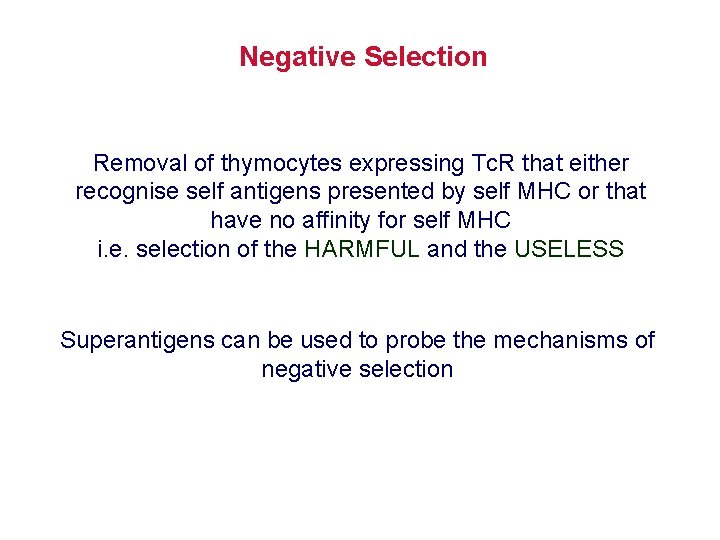
Negative Selection Removal of thymocytes expressing Tc. R that either recognise self antigens presented by self MHC or that have no affinity for self MHC i. e. selection of the HARMFUL and the USELESS Superantigens can be used to probe the mechanisms of negative selection

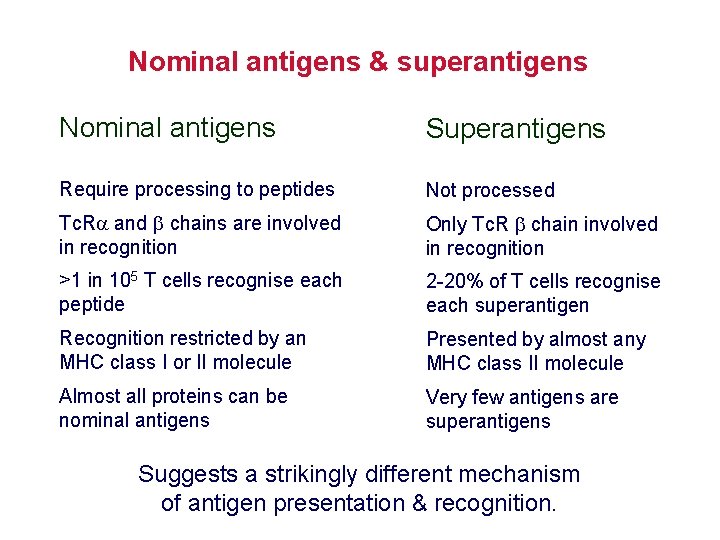
Nominal antigens & superantigens Nominal antigens Superantigens Require processing to peptides Not processed Tc. R and chains are involved in recognition Only Tc. R chain involved in recognition >1 in 105 T cells recognise each peptide 2 -20% of T cells recognise each superantigen Recognition restricted by an MHC class I or II molecule Presented by almost any MHC class II molecule Almost all proteins can be nominal antigens Very few antigens are superantigens Suggests a strikingly different mechanism of antigen presentation & recognition.

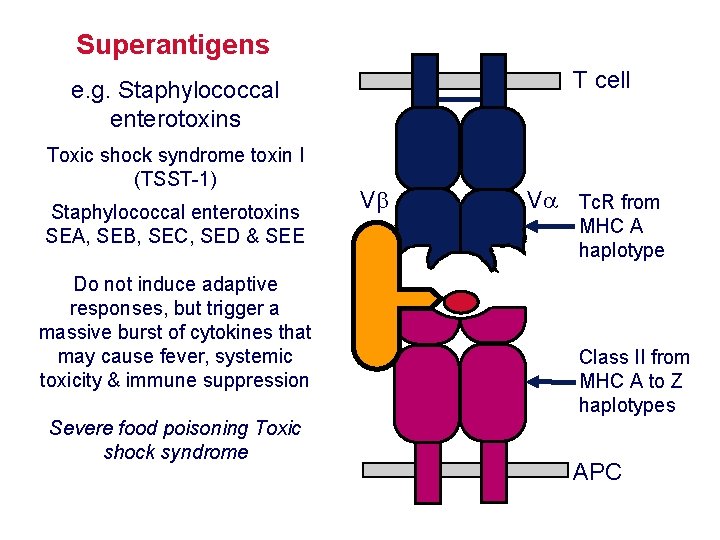
Superantigens T cell e. g. Staphylococcal enterotoxins Toxic shock syndrome toxin I (TSST-1) Staphylococcal enterotoxins SEA, SEB, SEC, SED & SEE Do not induce adaptive responses, but trigger a massive burst of cytokines that may cause fever, systemic toxicity & immune suppression Severe food poisoning Toxic shock syndrome V V Tc. R from MHC A haplotype Class II from MHC A to Z haplotypes APC

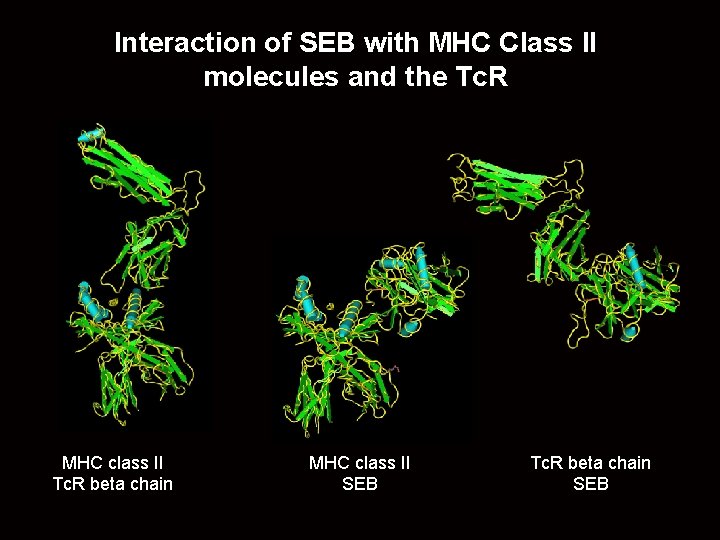
Interaction of SEB with MHC Class II molecules and the Tc. R MHC class II Tc. R beta chain MHC class II SEB Tc. R beta chain SEB

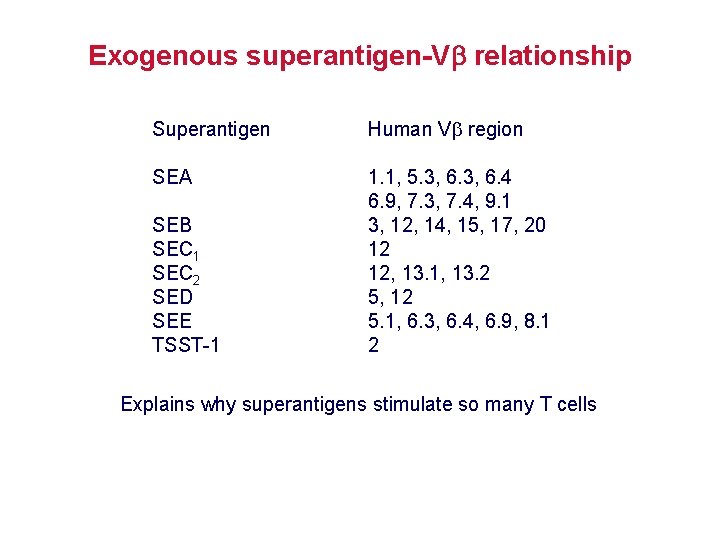
Exogenous superantigen-V relationship Superantigen Human V region SEA 1. 1, 5. 3, 6. 4 6. 9, 7. 3, 7. 4, 9. 1 3, 12, 14, 15, 17, 20 12 12, 13. 1, 13. 2 5, 12 5. 1, 6. 3, 6. 4, 6. 9, 8. 1 2 SEB SEC 1 SEC 2 SED SEE TSST-1 Explains why superantigens stimulate so many T cells

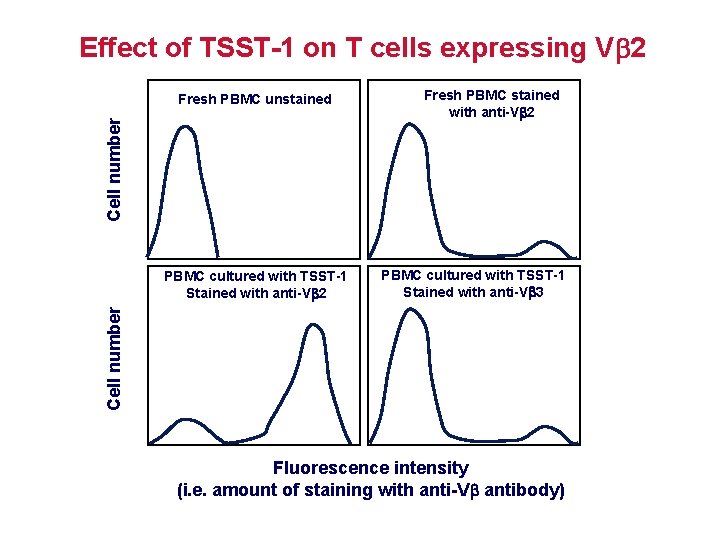
Effect of TSST-1 on T cells expressing V 2 Cell number Fresh PBMC unstained PBMC cultured with TSST-1 Stained with anti-V 3 Cell number PBMC cultured with TSST-1 Stained with anti-V 2 Fresh PBMC stained with anti-V 2 Fluorescence intensity (i. e. amount of staining with anti-V antibody)

Other exogenous superantigens Bacterial exoproteins Staphylococcal exfoliative toxins Streptococcus pyogenes erythrogenic toxins A & C (? Streptococcal M protein? ) Yersinia enterocolitica superantigen Clostridium perfingens superantigen Mycoplasma arthritidis mitogen

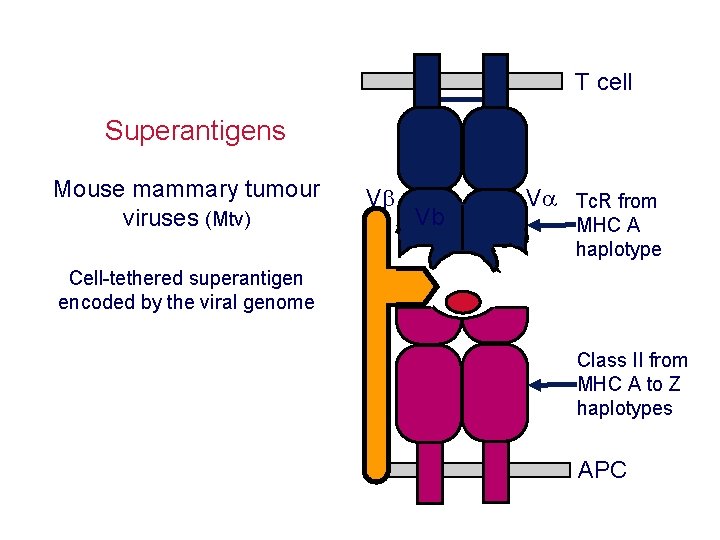
T cell Superantigens Mouse mammary tumour viruses (Mtv) V Vb V Tc. R from MHC A haplotype Cell-tethered superantigen encoded by the viral genome Class II from MHC A to Z haplotypes APC

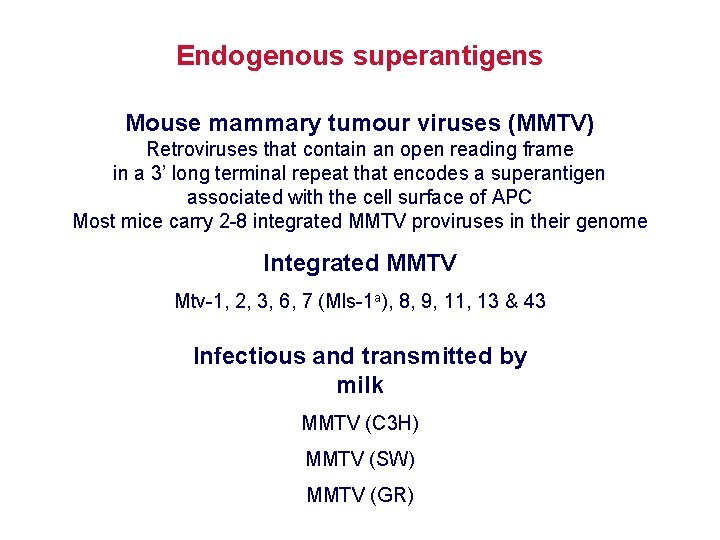
Endogenous superantigens Mouse mammary tumour viruses (MMTV) Retroviruses that contain an open reading frame in a 3’ long terminal repeat that encodes a superantigen associated with the cell surface of APC Most mice carry 2 -8 integrated MMTV proviruses in their genome Integrated MMTV Mtv-1, 2, 3, 6, 7 (Mls-1 a), 8, 9, 11, 13 & 43 Infectious and transmitted by milk MMTV (C 3 H) MMTV (SW) MMTV (GR)

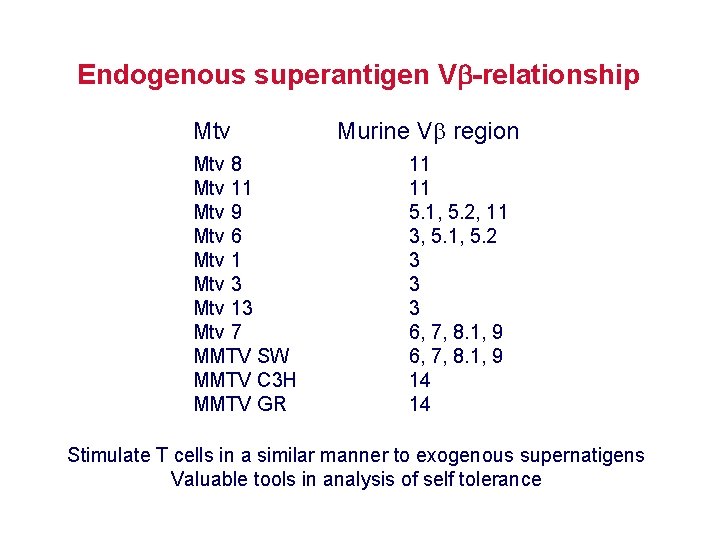
Endogenous superantigen V -relationship Mtv 8 Mtv 11 Mtv 9 Mtv 6 Mtv 1 Mtv 3 Mtv 13 Mtv 7 MMTV SW MMTV C 3 H MMTV GR Murine V region 11 11 5. 1, 5. 2, 11 3, 5. 1, 5. 2 3 3 3 6, 7, 8. 1, 9 14 14 Stimulate T cells in a similar manner to exogenous supernatigens Valuable tools in analysis of self tolerance

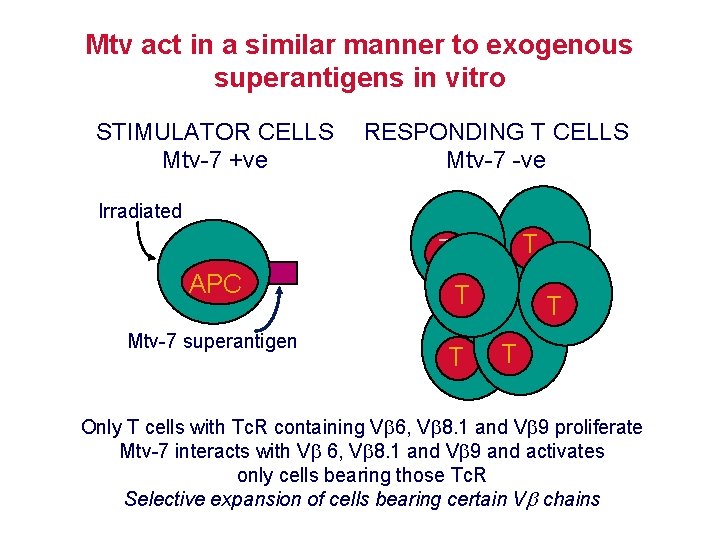
Mtv act in a similar manner to exogenous superantigens in vitro STIMULATOR CELLS Mtv-7 +ve RESPONDING T CELLS Mtv-7 -ve Irradiated T APC Mtv-7 superantigen T T T T Only T cells with Tc. R containing V 6, V 8. 1 and V 9 proliferate Mtv-7 interacts with V 6, V 8. 1 and V 9 and activates only cells bearing those Tc. R Selective expansion of cells bearing certain V chains

How do pathogens use superantigens? Unfocussed adaptive immune response activates cells of all specificities as well as those specific for the superantigens • Reduces the possibility that effective T cell clonal selection can eliminate the pathogen • Upon resolution, cells activated by the superantigen die, leaving the host immunosuppressed Transmission of infection

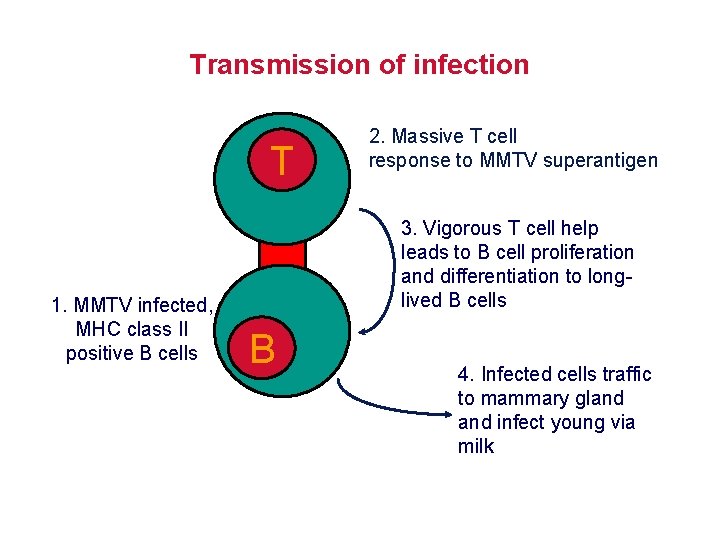
Transmission of infection T 1. MMTV infected, MHC class II positive B cells 2. Massive T cell response to MMTV superantigen 3. Vigorous T cell help leads to B cell proliferation and differentiation to longlived B cells B 4. Infected cells traffic to mammary gland infect young via milk

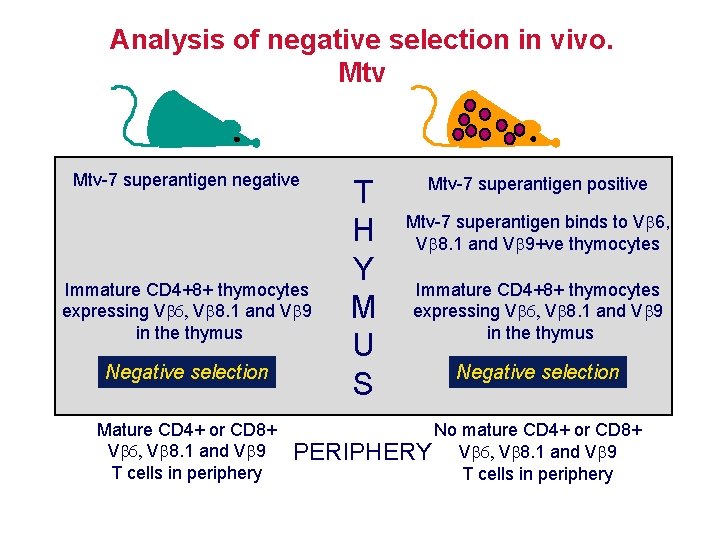
Analysis of negative selection in vivo. Mtv-7 superantigen negative Immature CD 4+8+ thymocytes expressing V V 8. 1 and V 9 in the thymus Negative selection Mature CD 4+ or CD 8+ V V 8. 1 and V 9 T cells in periphery T H Y M U S Mtv-7 superantigen positive Mtv-7 superantigen binds to V 6, V 8. 1 and V 9+ve thymocytes Immature CD 4+8+ thymocytes expressing V V 8. 1 and V 9 in the thymus Negative selection No mature CD 4+ or CD 8+ PERIPHERY V V 8. 1 and V 9 T cells in periphery

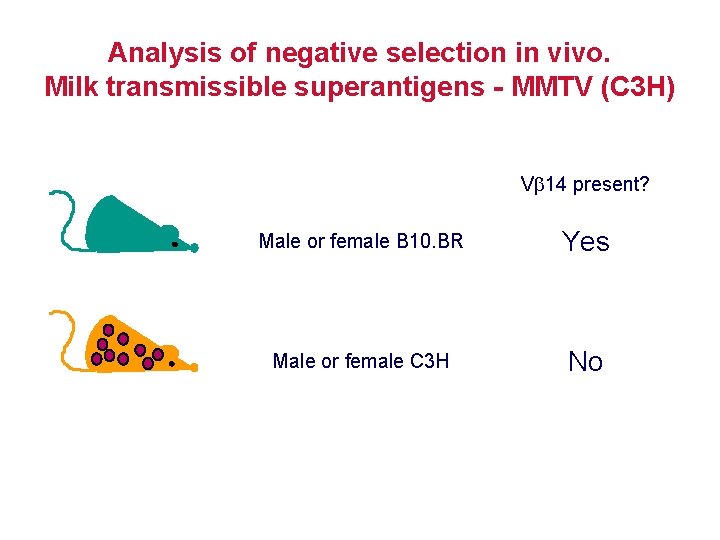
Analysis of negative selection in vivo. Milk transmissible superantigens - MMTV (C 3 H) V 14 present? Male or female B 10. BR Yes Male or female C 3 H No

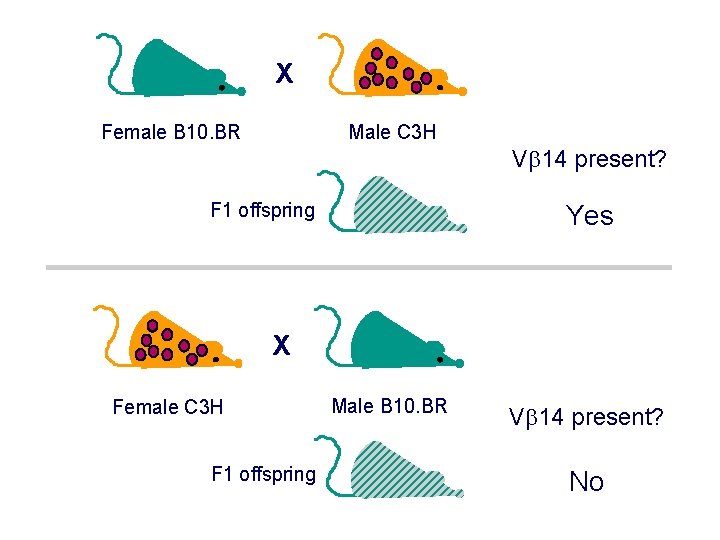
X Female B 10. BR Male C 3 H V 14 present? F 1 offspring Yes X Female C 3 H F 1 offspring Male B 10. BR V 14 present? No

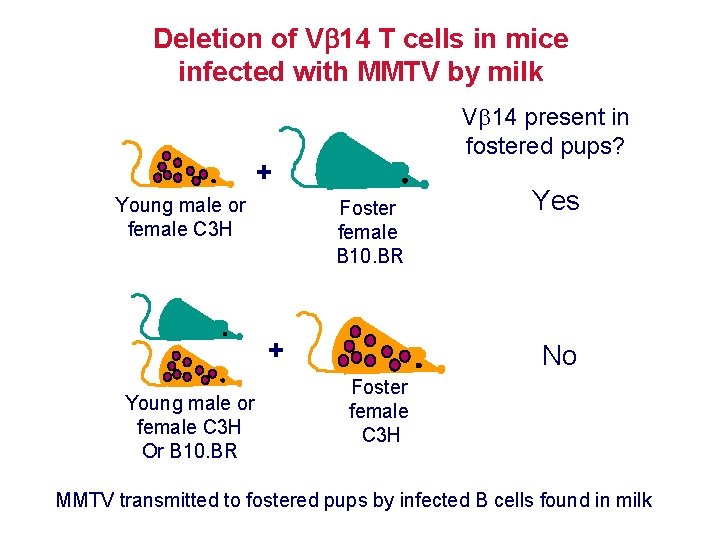
Deletion of V 14 T cells in mice infected with MMTV by milk V 14 present in fostered pups? + Young male or female C 3 H Foster female B 10. BR + Young male or female C 3 H Or B 10. BR Yes No Foster female C 3 H MMTV transmitted to fostered pups by infected B cells found in milk

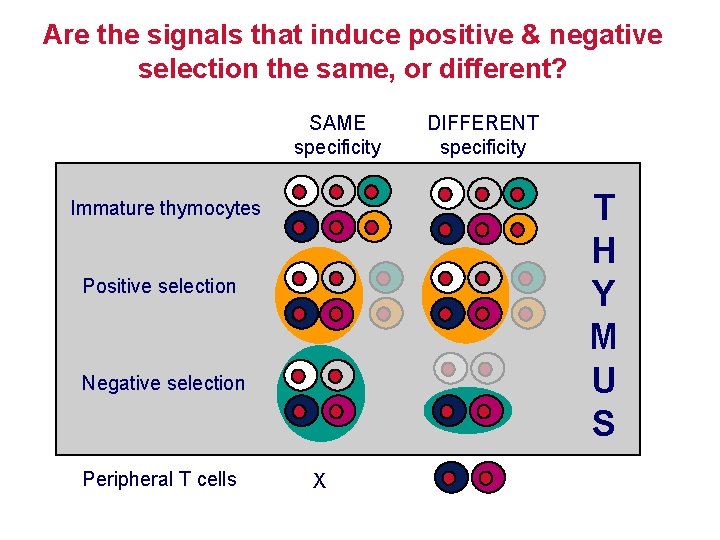
Are the signals that induce positive & negative selection the same, or different? SAME specificity T H Y M U S Immature thymocytes Positive selection Negative selection Peripheral T cells DIFFERENT specificity X

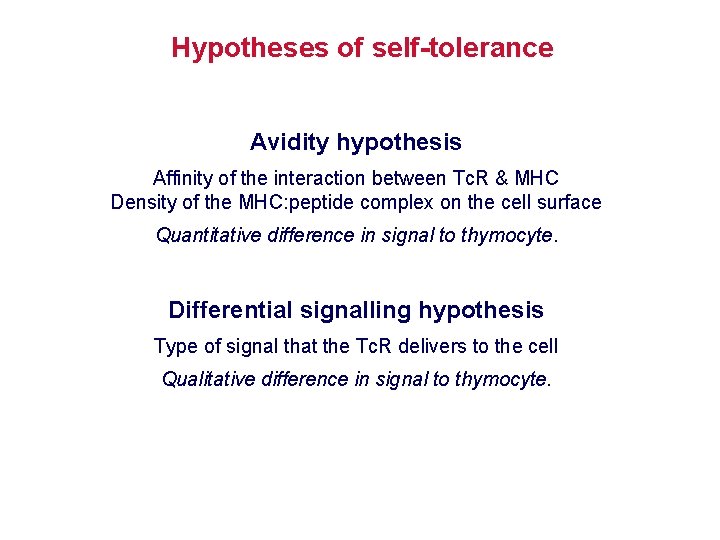
Hypotheses of self-tolerance Avidity hypothesis Affinity of the interaction between Tc. R & MHC Density of the MHC: peptide complex on the cell surface Quantitative difference in signal to thymocyte. Differential signalling hypothesis Type of signal that the Tc. R delivers to the cell Qualitative difference in signal to thymocyte.

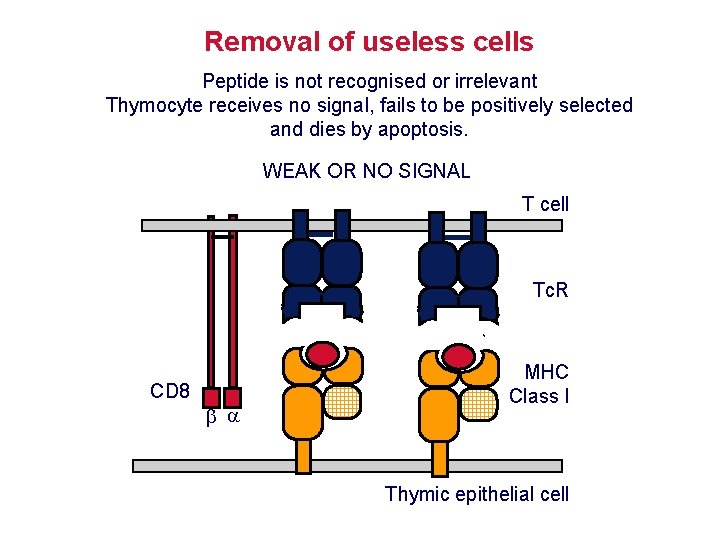
Removal of useless cells Peptide is not recognised or irrelevant Thymocyte receives no signal, fails to be positively selected and dies by apoptosis. WEAK OR NO SIGNAL T cell Tc. R CD 8 MHC Class I Thymic epithelial cell

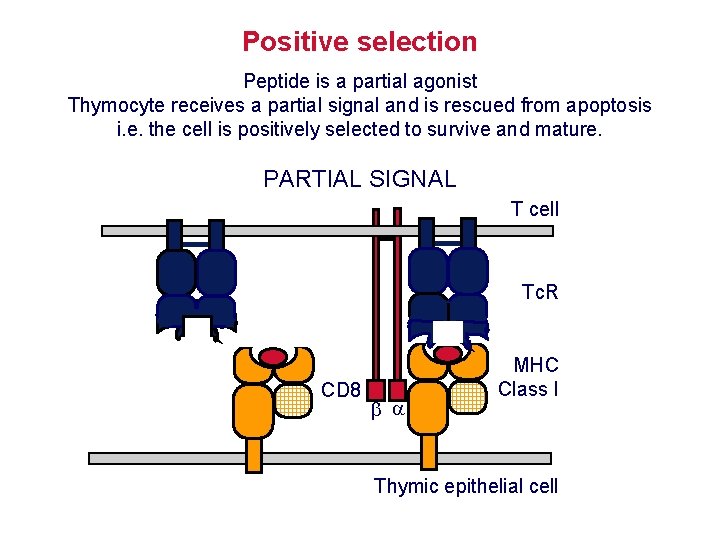
Positive selection Peptide is a partial agonist Thymocyte receives a partial signal and is rescued from apoptosis i. e. the cell is positively selected to survive and mature. PARTIAL SIGNAL T cell Tc. R CD 8 MHC Class I Thymic epithelial cell

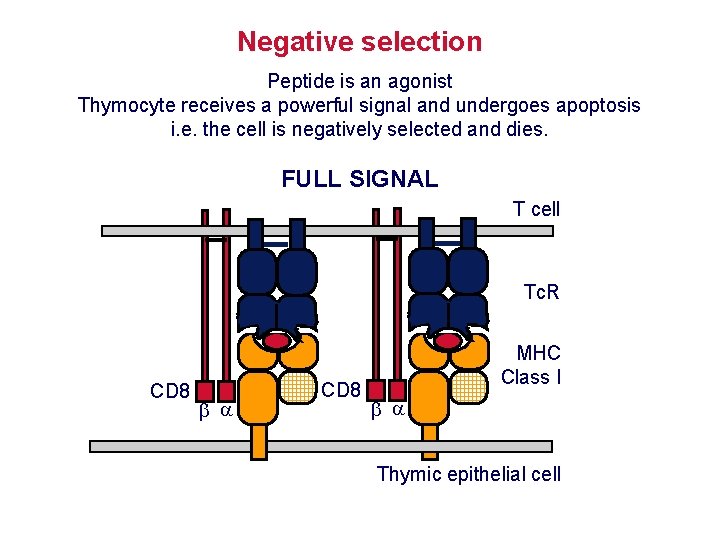
Negative selection Peptide is an agonist Thymocyte receives a powerful signal and undergoes apoptosis i. e. the cell is negatively selected and dies. FULL SIGNAL T cell Tc. R CD 8 MHC Class I Thymic epithelial cell

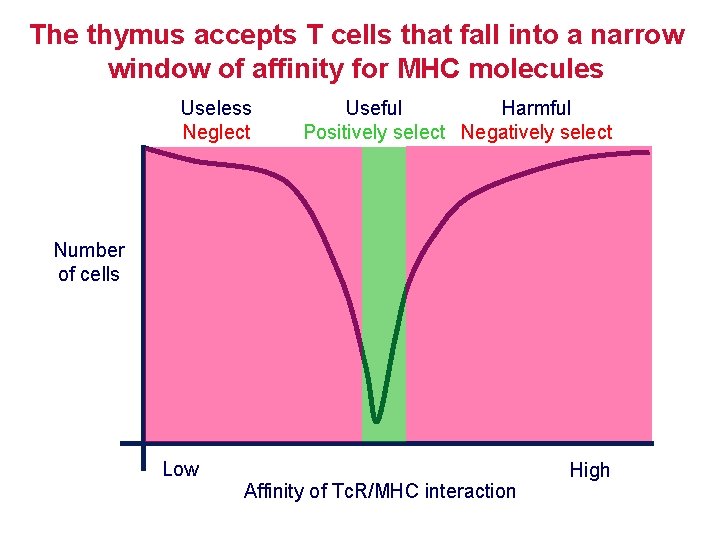
The thymus accepts T cells that fall into a narrow window of affinity for MHC molecules Useless Neglect Useful Harmful Positively select Negatively select Number of cells Low Affinity of Tc. R/MHC interaction High

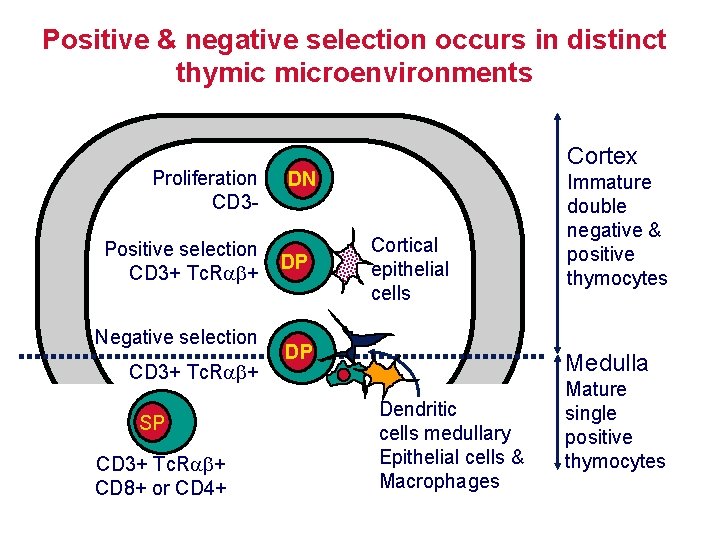
Positive & negative selection occurs in distinct thymic microenvironments Proliferation CD 3 Positive selection CD 3+ Tc. R + Negative selection CD 3+ Tc. R + SP CD 3+ Tc. R + CD 8+ or CD 4+ Cortex DN DP Cortical epithelial cells DP Immature double negative & positive thymocytes Medulla Dendritic cells medullary Epithelial cells & Macrophages Mature single positive thymocytes

How accurate are these models of positive and negative selection? Positive selection: Relied on very complex chimera experiments Relied on proof of MHC restriction as an outcome which is tested in an ‘unnatural’ response using MHC mismatched presenting cells Negative selection: Relied on exceptionally powerful superantigens operating outside the normal mechanisms of antigen recognition

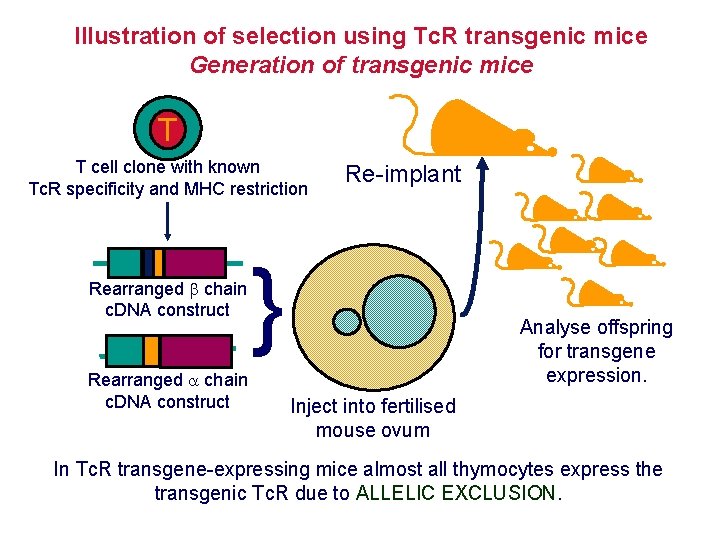
Illustration of selection using Tc. R transgenic mice Generation of transgenic mice T T cell clone with known Tc. R specificity and MHC restriction Rearranged chain c. DNA construct Re-implant } Analyse offspring for transgene expression. Inject into fertilised mouse ovum In Tc. R transgene-expressing mice almost all thymocytes express the transgenic Tc. R due to ALLELIC EXCLUSION.

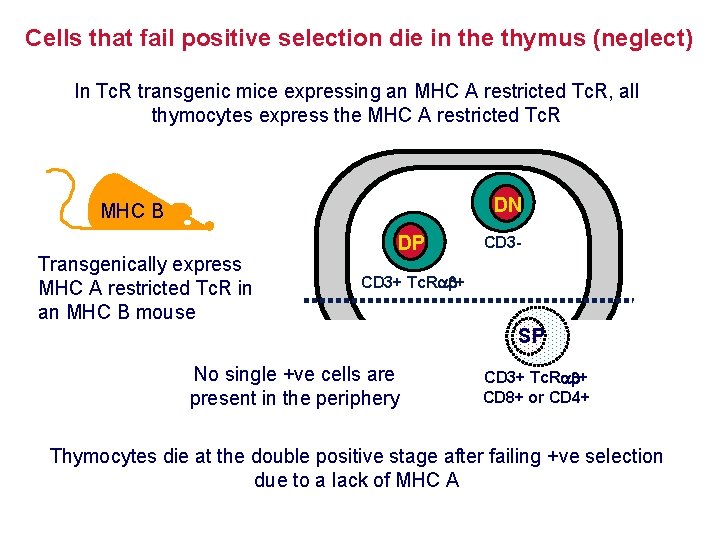
Cells that fail positive selection die in the thymus (neglect) In Tc. R transgenic mice expressing an MHC A restricted Tc. R, all thymocytes express the MHC A restricted Tc. R DN MHC B Transgenically express MHC A restricted Tc. R in an MHC B mouse DP CD 3 - CD 3+ Tc. R + SP No single +ve cells are present in the periphery CD 3+ Tc. R + CD 8+ or CD 4+ Thymocytes die at the double positive stage after failing +ve selection due to a lack of MHC A

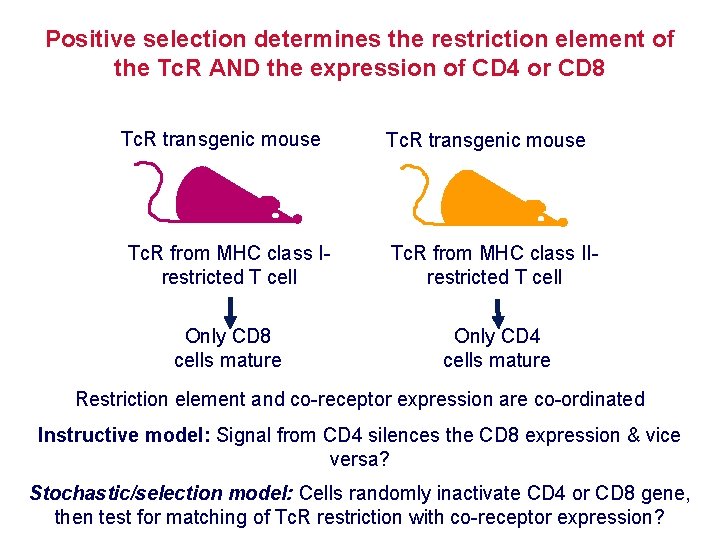
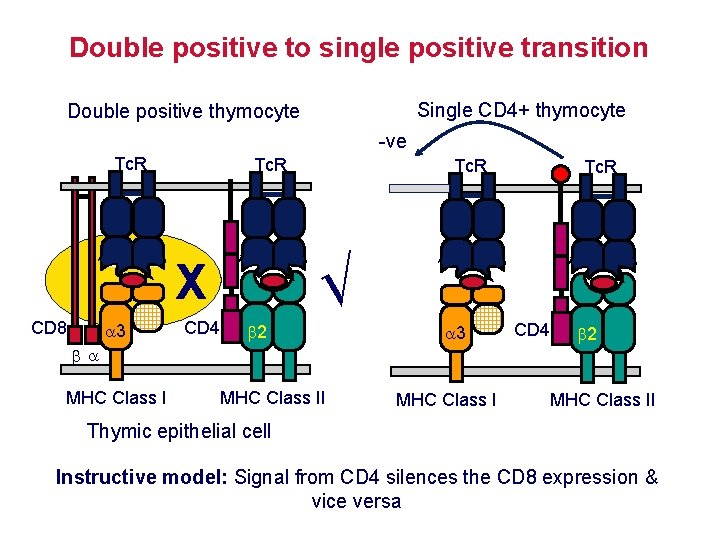
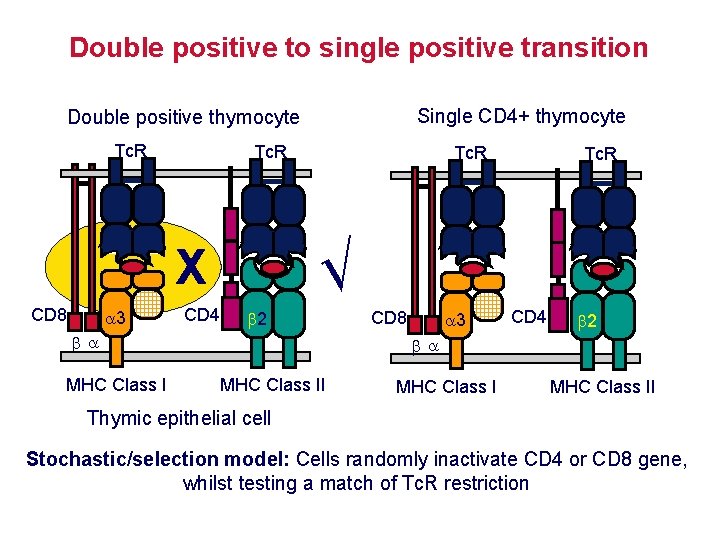
Positive selection determines the restriction element of the Tc. R AND the expression of CD 4 or CD 8 Tc. R transgenic mouse Tc. R from MHC class Irestricted T cell Tc. R from MHC class IIrestricted T cell Only CD 8 cells mature Only CD 4 cells mature Restriction element and co-receptor expression are co-ordinated Instructive model: Signal from CD 4 silences the CD 8 expression & vice versa? Stochastic/selection model: Cells randomly inactivate CD 4 or CD 8 gene, then test for matching of Tc. R restriction with co-receptor expression?

Double positive to single positive transition Single CD 4+ thymocyte Double positive thymocyte -ve Tc. R 3 CD 4 2 MHC Class I Tc. R √ X CD 8 Tc. R CD 8 3 CD 4 2 MHC Class II Thymic epithelial cell Instructive model: Signal from CD 4 silences the CD 8 expression & vice versa

Double positive to single positive transition Single CD 4+ thymocyte Double positive thymocyte Tc. R 3 CD 4 2 MHC Class I Tc. R √ X CD 8 Tc. R 3 CD 8 CD 4 2 MHC Class II Thymic epithelial cell Stochastic/selection model: Cells randomly inactivate CD 4 or CD 8 gene, whilst testing a match of Tc. R restriction

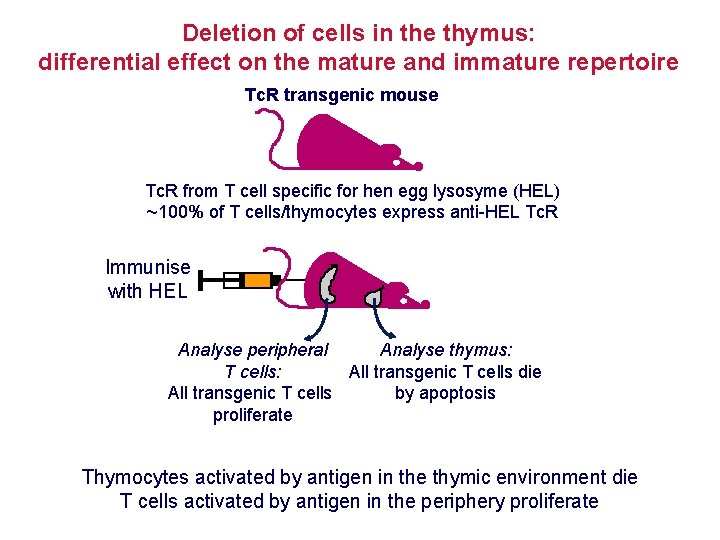
Deletion of cells in the thymus: differential effect on the mature and immature repertoire Tc. R transgenic mouse Tc. R from T cell specific for hen egg lysosyme (HEL) ~100% of T cells/thymocytes express anti-HEL Tc. R Immunise with HEL Analyse peripheral Analyse thymus: T cells: All transgenic T cells die All transgenic T cells by apoptosis proliferate Thymocytes activated by antigen in the thymic environment die T cells activated by antigen in the periphery proliferate

How can the thymus express all self antigens – including self antigens only made by specialised tissues? How do we become self tolerant to these antigens?

Nature Immunology November 2001

Promiscuous expression of tissue-specific genes by medullary thymic epithelial cells

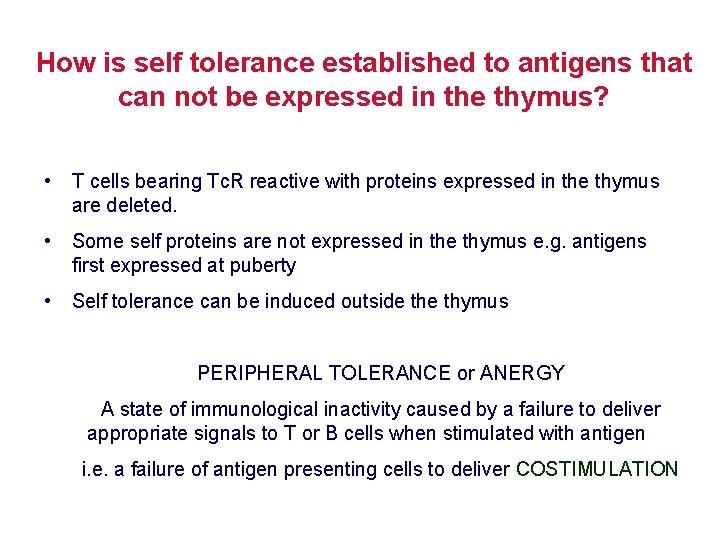
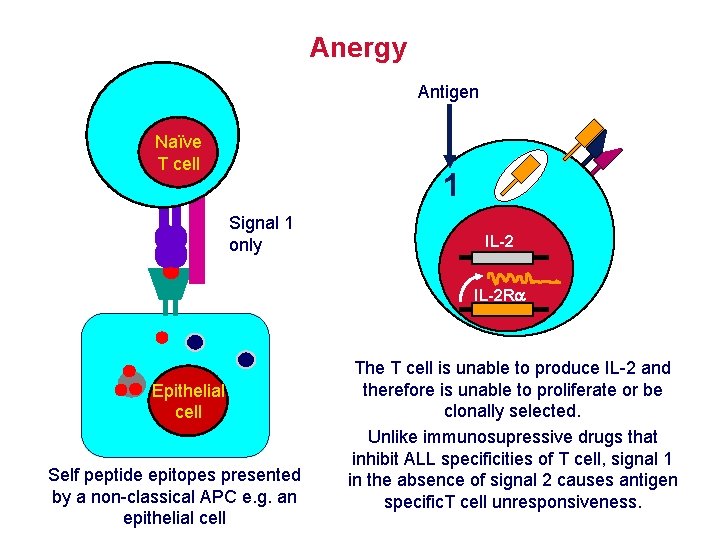
How is self tolerance established to antigens that can not be expressed in the thymus? • T cells bearing Tc. R reactive with proteins expressed in the thymus are deleted. • Some self proteins are not expressed in the thymus e. g. antigens first expressed at puberty • Self tolerance can be induced outside thymus PERIPHERAL TOLERANCE or ANERGY A state of immunological inactivity caused by a failure to deliver appropriate signals to T or B cells when stimulated with antigen i. e. a failure of antigen presenting cells to deliver COSTIMULATION

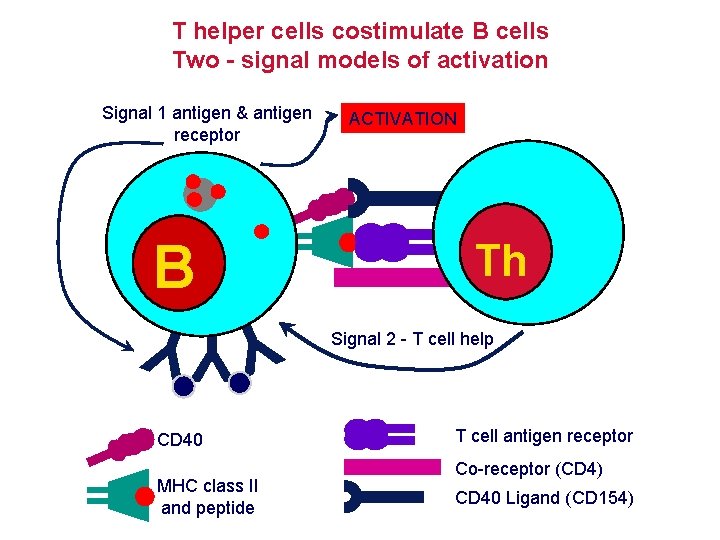
T helper cells costimulate B cells Two - signal models of activation Signal 1 antigen & antigen receptor B YYY CD 40 MHC class II and peptide ACTIVATION Th Signal 2 - T cell help T cell antigen receptor Co-receptor (CD 4) CD 40 Ligand (CD 154)

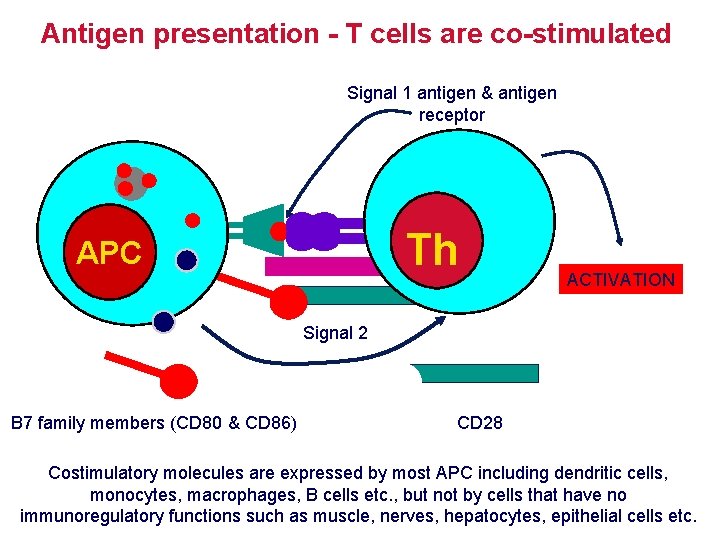
Antigen presentation - T cells are co-stimulated Signal 1 antigen & antigen receptor Th APC ACTIVATION Signal 2 B 7 family members (CD 80 & CD 86) CD 28 Costimulatory molecules are expressed by most APC including dendritic cells, monocytes, macrophages, B cells etc. , but not by cells that have no immunoregulatory functions such as muscle, nerves, hepatocytes, epithelial cells etc.

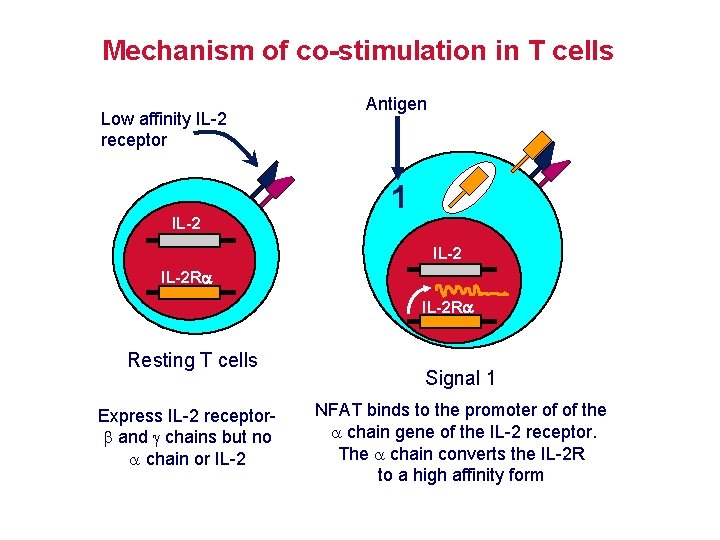
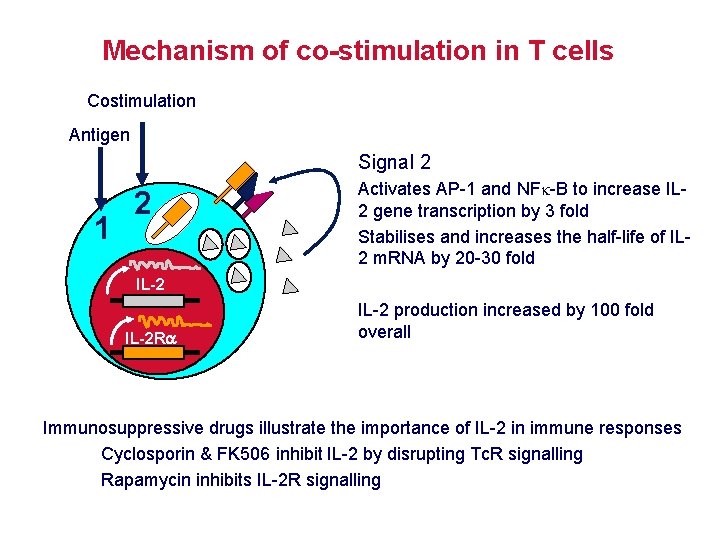
Mechanism of co-stimulation in T cells Low affinity IL-2 receptor Antigen 1 IL-2 R Resting T cells Express IL-2 receptor and chains but no chain or IL-2 Signal 1 NFAT binds to the promoter of of the chain gene of the IL-2 receptor. The chain converts the IL-2 R to a high affinity form

Mechanism of co-stimulation in T cells Costimulation Antigen Signal 2 1 2 Activates AP-1 and NFk-B to increase IL 2 gene transcription by 3 fold Stabilises and increases the half-life of IL 2 m. RNA by 20 -30 fold IL-2 R IL-2 production increased by 100 fold overall Immunosuppressive drugs illustrate the importance of IL-2 in immune responses Cyclosporin & FK 506 inhibit IL-2 by disrupting Tc. R signalling Rapamycin inhibits IL-2 R signalling

Anergy Antigen Naïve T cell 1 Signal 1 only IL-2 R Epithelial cell Self peptide epitopes presented by a non-classical APC e. g. an epithelial cell The T cell is unable to produce IL-2 and therefore is unable to proliferate or be clonally selected. Unlike immunosupressive drugs that inhibit ALL specificities of T cell, signal 1 in the absence of signal 2 causes antigen specific. T cell unresponsiveness.

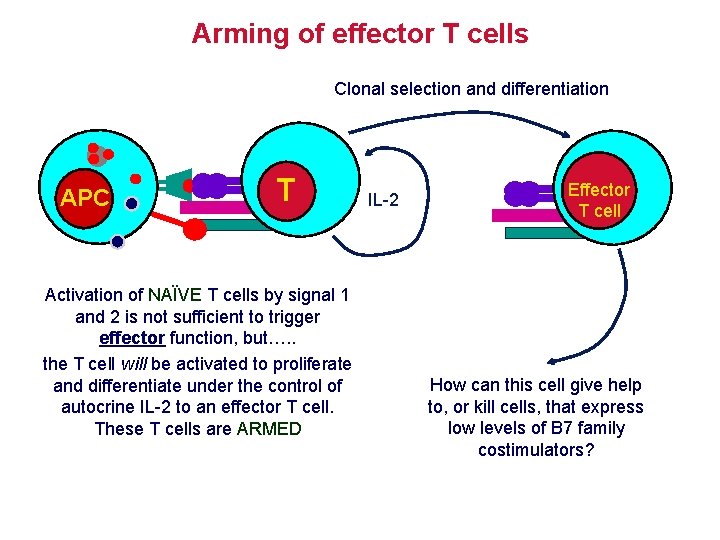
Arming of effector T cells Clonal selection and differentiation APC T Activation of NAÏVE T cells by signal 1 and 2 is not sufficient to trigger effector function, but…. . the T cell will be activated to proliferate and differentiate under the control of autocrine IL-2 to an effector T cell. These T cells are ARMED IL-2 Effector T cell How can this cell give help to, or kill cells, that express low levels of B 7 family costimulators?

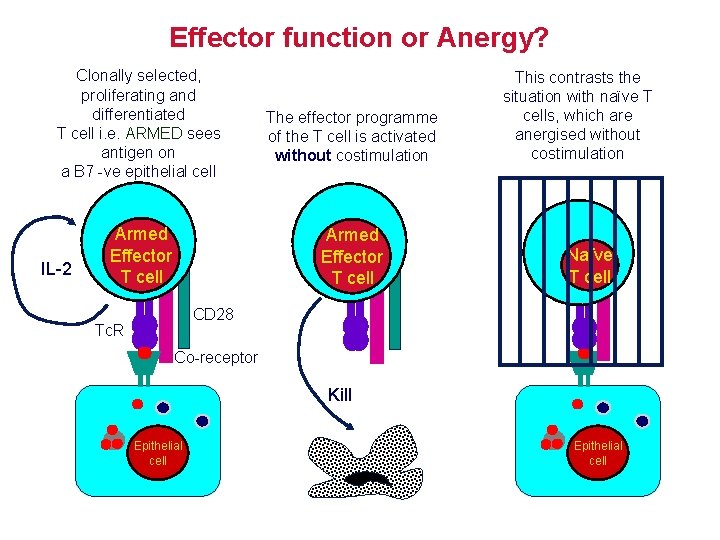
Effector function or Anergy? Clonally selected, proliferating and differentiated T cell i. e. ARMED sees antigen on a B 7 -ve epithelial cell IL-2 Armed Effector T cell The effector programme of the T cell is activated without costimulation Armed Effector T cell This contrasts the situation with naïve T cells, which are anergised without costimulation Naïve T cell CD 28 Tc. R Co-receptor Kill Epithelial cell

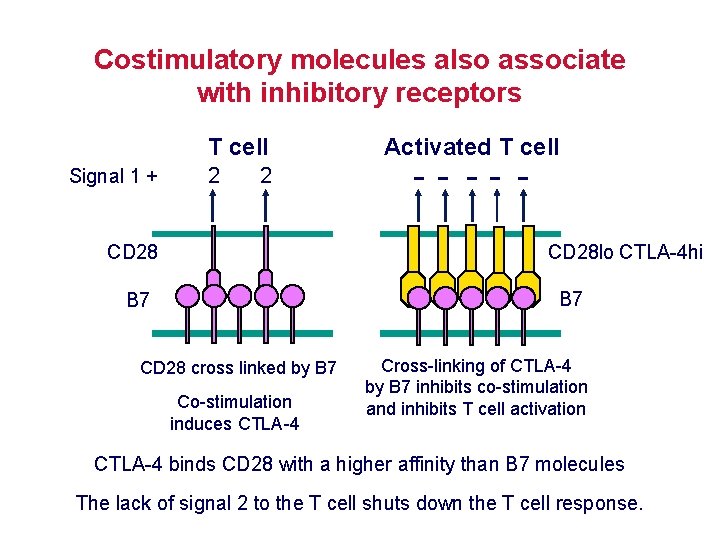
Costimulatory molecules also associate with inhibitory receptors T cell Signal 1 + 2 2 Activated T cell -- -- - CD 28 lo CTLA-4 hi CD 28 B 7 CD 28 cross linked by B 7 Co-stimulation induces CTLA-4 Cross-linking of CTLA-4 by B 7 inhibits co-stimulation and inhibits T cell activation CTLA-4 binds CD 28 with a higher affinity than B 7 molecules The lack of signal 2 to the T cell shuts down the T cell response.

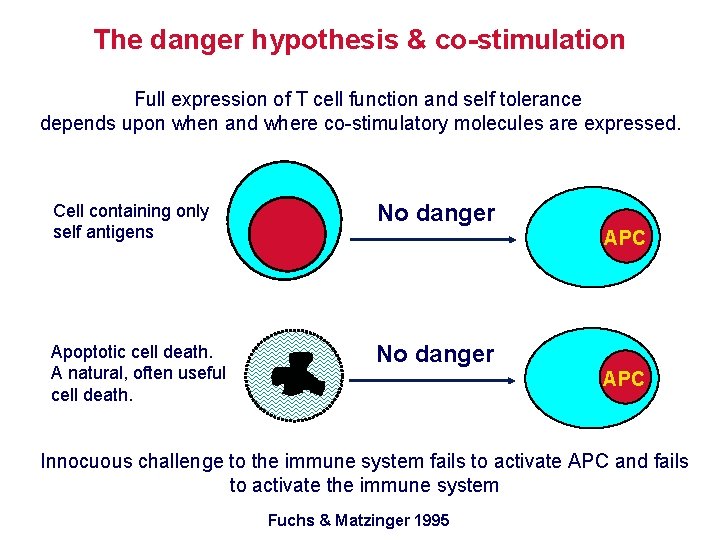
The danger hypothesis & co-stimulation Full expression of T cell function and self tolerance depends upon when and where co-stimulatory molecules are expressed. Cell containing only self antigens No danger Apoptotic cell death. A natural, often useful cell death. No danger APC Innocuous challenge to the immune system fails to activate APC and fails to activate the immune system Fuchs & Matzinger 1995

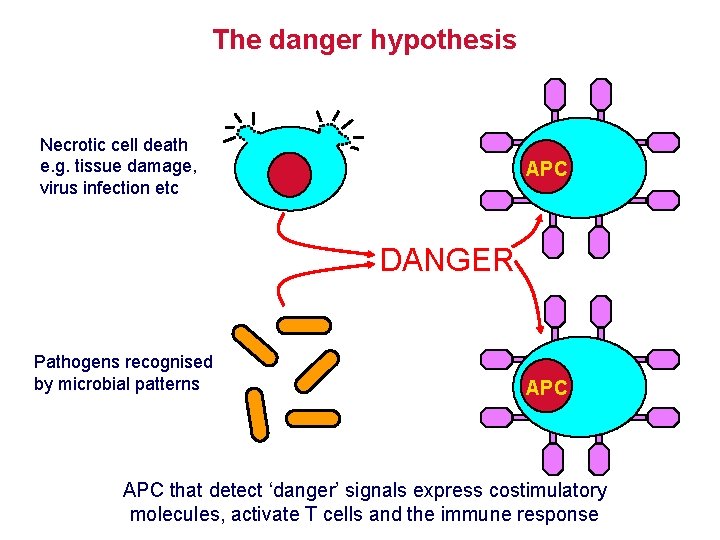
The danger hypothesis Necrotic cell death e. g. tissue damage, virus infection etc APC DANGER Pathogens recognised by microbial patterns APC that detect ‘danger’ signals express costimulatory molecules, activate T cells and the immune response

How the danger hypothesis suggests a review of immunological dogma • Antigens induce tolerance or immunity depending upon the ability of the immune system to sense them as ‘dangererous’, and not by sensing whether they are self or ‘non-self’. • There is no window for tolerance induction in neonates - if a ‘danger signal’ is received, the neonatal immune system will respond • Neonatal T cells are not intrinsically tolerisable but the natural antiinflammatory nature of the neonatal environment predisposes to tolerance • Apoptosis, the ‘non-dangerous’ death of self cells may prevent autoimmunity when old or surplus cells are disposed of. • Suggests that tolerance is the default pathway of the immune system on encountering antigens. • Explains why immunisations require adjuvants to stimulate cues of danger such as cytokines or costimulatory molecule expression. Doesn’t exclude self-nonself discrimination, but the danger hypothesis will be very hard to disprove experimentally.