Topic 7 Equilibrium 7 Dynamic Equilibrium 1 Le

Topic 7 - Equilibrium 7 – Dynamic Equilibrium 1

Le Chatelier’s Principle Objectives: Understand the impact of Le Chatelier’s Principle Use Le Chatelier’s Principle to explain the effect of changes on a system at equilibrium Complete some short experiments to observe the effect of Le Chatelier’s Principle
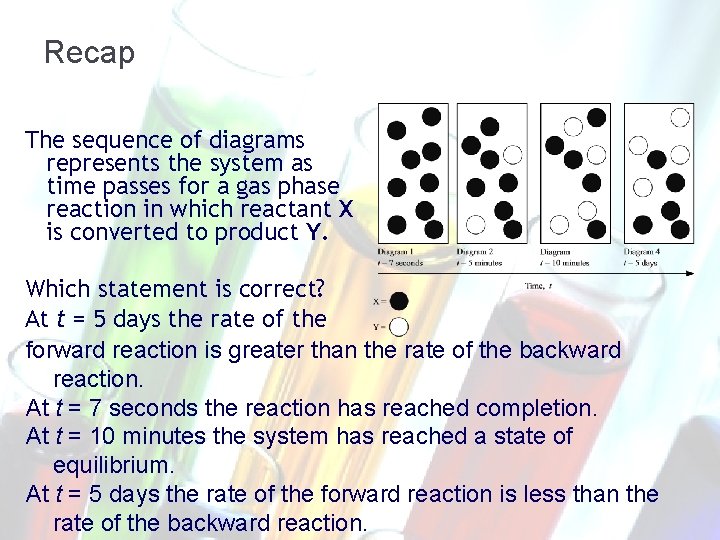
Recap The sequence of diagrams represents the system as time passes for a gas phase reaction in which reactant X is converted to product Y. Which statement is correct? At t = 5 days the rate of the forward reaction is greater than the rate of the backward reaction. At t = 7 seconds the reaction has reached completion. At t = 10 minutes the system has reached a state of equilibrium. At t = 5 days the rate of the forward reaction is less than the rate of the backward reaction.
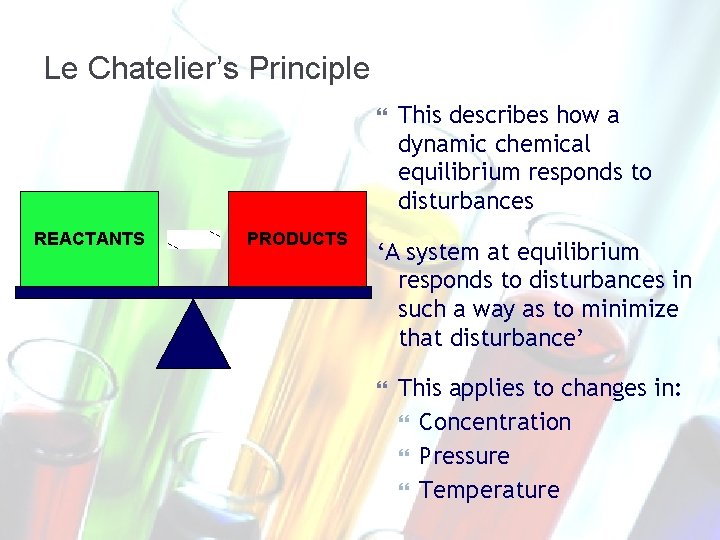
Le Chatelier’s Principle REACTANTS PRODUCTS This describes how a dynamic chemical equilibrium responds to disturbances ‘A system at equilibrium responds to disturbances in such a way as to minimize that disturbance’ This applies to changes in: Concentration Pressure Temperature
![Changes in Concentration Decrease in [Reactant] or increase in [Product] REA CTA NTS PRO Changes in Concentration Decrease in [Reactant] or increase in [Product] REA CTA NTS PRO](http://slidetodoc.com/presentation_image_h2/b35f95affced1da10208557b5c99f8c2/image-5.jpg)
Changes in Concentration Decrease in [Reactant] or increase in [Product] REA CTA NTS PRO DUC � Equilibrium Ethanol CH 3 CH 2 OH(aq) TS Equilibrium shifts to the left This has the effect of increasing [Reactant] and decreasing [Product] constant is not affected + Ethanoic Acid + CH 3 CO 2 H(aq) ⇌ ⇌ Ethyl ethanoate + CH 3 CH 2 OOCCH 3(aq) + water H 2 O(l)
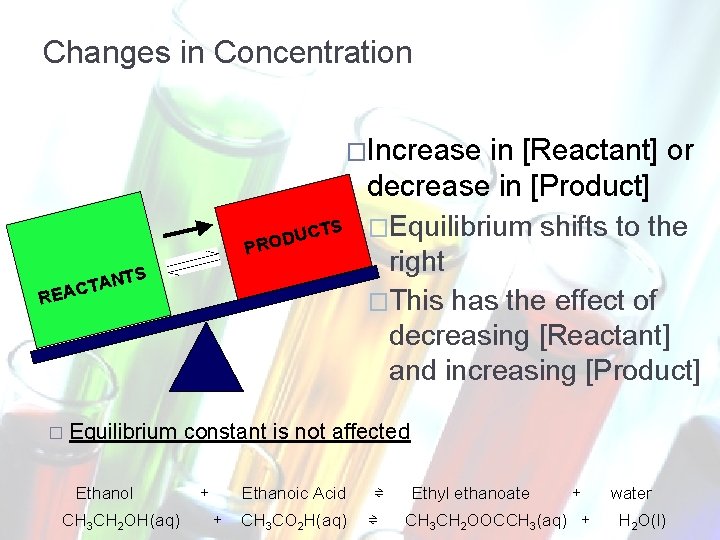
Changes in Concentration �Increase CTS U D RO P TS TAN C A E R � Equilibrium Ethanol CH 3 CH 2 OH(aq) in [Reactant] or decrease in [Product] �Equilibrium shifts to the right �This has the effect of decreasing [Reactant] and increasing [Product] constant is not affected + Ethanoic Acid + CH 3 CO 2 H(aq) ⇌ ⇌ Ethyl ethanoate + CH 3 CH 2 OOCCH 3(aq) + water H 2 O(l)
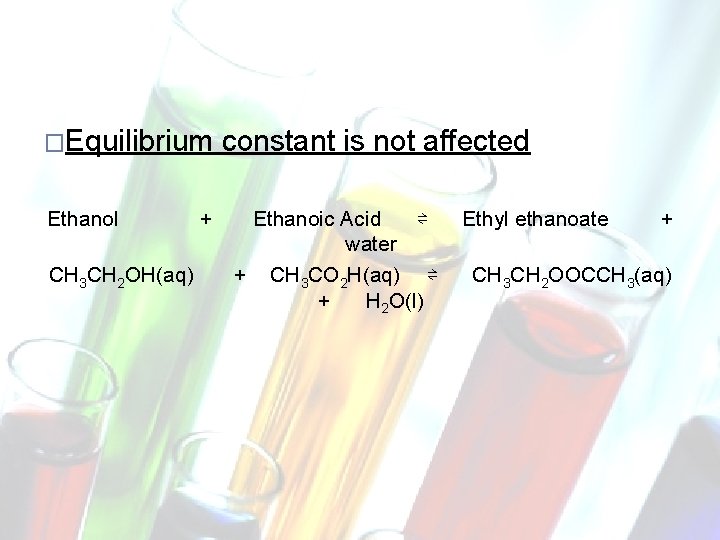
�Equilibrium Ethanol CH 3 CH 2 OH(aq) + constant is not affected Ethanoic Acid ⇌ water + CH 3 CO 2 H(aq) ⇌ + H 2 O(l) Ethyl ethanoate + CH 3 CH 2 OOCCH 3(aq)

Changes in Pressure Increasing Pressure: Decreasing Pressure: Shifts equilibrium to the side with fewest gas molecules This has the effect of reducing the pressure increase Shifts equilibrium to the side with more gas molecules This has the effect of increasing the pressure Equilibrium constant is not affected 2 N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) H = -91. 8 k. J mol-1
Changes in Temperature Exothermic Reaction: Increasing temperature shifts equilibrium to left Decreasing temperature shifts equilibrium to the right This is the ‘hot’ side of the reaction, so temperature increases Endothermic Reaction: Increasing temperature shifts equilibrium to the right This is the ‘cold’ side of the reaction, so temperature decreases Decreasing temperature shifts equilibrium to the left The left is the ‘cold’ side of the reaction, so temperature decreases This is the ‘hot’ side of the reaction, so temperature increases Equilibrium constant is affected Exothermic Reaction: Higher Temp Lower Kc; Lower Temp Higher Kc Endothermic Reaction: Higher Temp Higher Kc; Lower Temp Lower Kc 2 N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) H = -91. 8 k. J mol-1
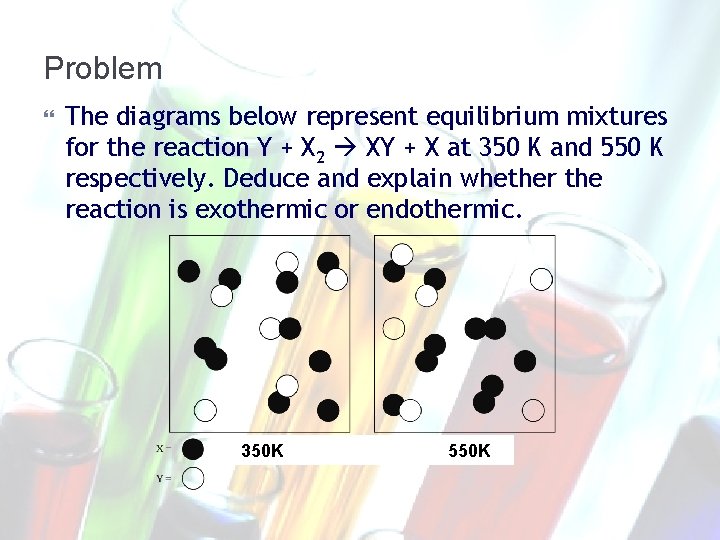
Problem The diagrams below represent equilibrium mixtures for the reaction Y + X 2 XY + X at 350 K and 550 K respectively. Deduce and explain whether the reaction is exothermic or endothermic. 350 K 550 K

Recap Le Chatelier’s Principle explains how equilibrium systems respond to changes Any disturbance to a system will be minimised but not eliminated.
- Slides: 11