Topic 4 2 d Plastics PLASTICS All plastics













- Slides: 21

Topic 4. 2 d Plastics PLASTICS. All plastics were soft and mouldable during their production - that's why they're called plastics. The Greek word plasticós means "to mould. " You can form nearly any object out of plastics from bristles on toothbrushes to bullet-proof vests to fibres for making textiles for clothes. Soon, tiny plastic projectiles may be used as carriers of vaccine, making it possible to swallow the vaccine instead of getting an injection! Plastics are synthetic materials, which means that they are artificial, or manufactured. Synthesis means that "something is put together, " and synthetic materials are made of building blocks that are put together in factories. A monomer from oil - this one is the hydrocarbon ethylene. The building blocks for making plastics are small organic molecules - molecules that contain carbon along with other substances. They generally come from oil (petroleum) or natural gas, but they can also come from other organic materials such as wood fibres, corn, or banana peels! Each of these small molecules is known as a monomer ("one part") because it's capable of joining with other monomers to form very long molecule chains called polymers ("many parts") during a A polymer - polyethylene - made of chemical reaction called ethylene monomers. polymerization. To visualize this, think of a single paper clip as a monomer, and all the paper clips in a box chained together as a polymer. http: //nobelprize. org/educational_games/chemistry/plastics/readmore. html

Topic 4. 2 d Plastics are polymers, but polymers don't have to be plastics. The way plastics are made is actually a way of imitating nature, which has created a huge number of polymers. Cellulose, the basic component of plant cell walls is a polymer, and so are all the proteins produced in your body and the proteins you eat. Another famous example of a polymer is DNA - the long molecule in the nuclei of your cells that carries all the genetic information about you. People have been using natural polymers, including silk, wool, cotton, wood, and leather for centuries. These products inspired chemists to try to create synthetic counterparts, which they have done with amazing success. q Plastics are synthetic materials derived from organic (carbon-containing) compounds. The most common sources for carbon compounds are oil (petroleum) and natural gas. q Plastics consist of polymers - long molecule chains - often mixed with other substances such as colouring agents and softeners. q The properties of a particular plastic depend on what the polymer chains look like, how they are bonded to each other, and which additives have been introduced. There are two groups of plastics: Thermoplastics, which melt when heated and can be remoulded easily. Thermosets, which can't melt or be remoulded. They crack or char when heated. http: //nobelprize. org/educational_games/chemistry/plastics/readmore. html

Types of plastics There are 2 main types of plastics n Thermosets - Made from long chain molecules which bond together strongly when the plastics are made. This means that they cannot be re-melted. Thermosets are rigid and non-flexible even at high temperatures. n 3 Thermoplastics - Also made from long chain molecules which are entangled but not bonded together. This means that they can be melted and re -formed. (“Thermo” = heat, “plastic” = able to be formed)

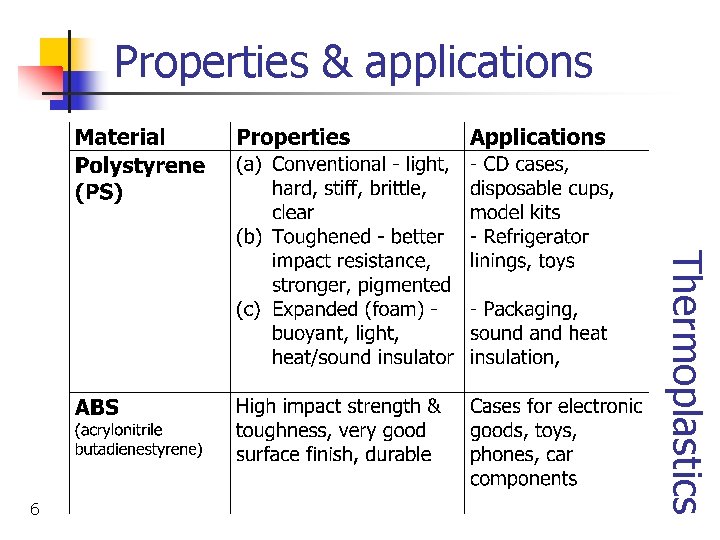
Properties & applications Thermosets 4

Properties & applications Thermoplastics 5

Properties & applications Thermoplastics 6

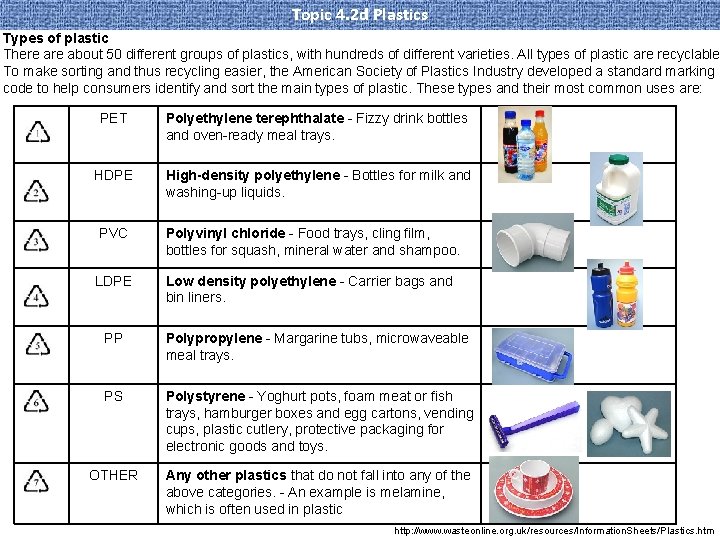
Topic 4. 2 d Plastics Types of plastic There about 50 different groups of plastics, with hundreds of different varieties. All types of plastic are recyclable To make sorting and thus recycling easier, the American Society of Plastics Industry developed a standard marking code to help consumers identify and sort the main types of plastic. These types and their most common uses are: PET Polyethylene terephthalate - Fizzy drink bottles and oven-ready meal trays. HDPE High-density polyethylene - Bottles for milk and washing-up liquids. PVC Polyvinyl chloride - Food trays, cling film, bottles for squash, mineral water and shampoo. LDPE Low density polyethylene - Carrier bags and bin liners. PP Polypropylene - Margarine tubs, microwaveable meal trays. PS Polystyrene - Yoghurt pots, foam meat or fish trays, hamburger boxes and egg cartons, vending cups, plastic cutlery, protective packaging for electronic goods and toys. OTHER Any other plastics that do not fall into any of the above categories. - An example is melamine, which is often used in plastic http: //www. wasteonline. org. uk/resources/Information. Sheets/Plastics. htm

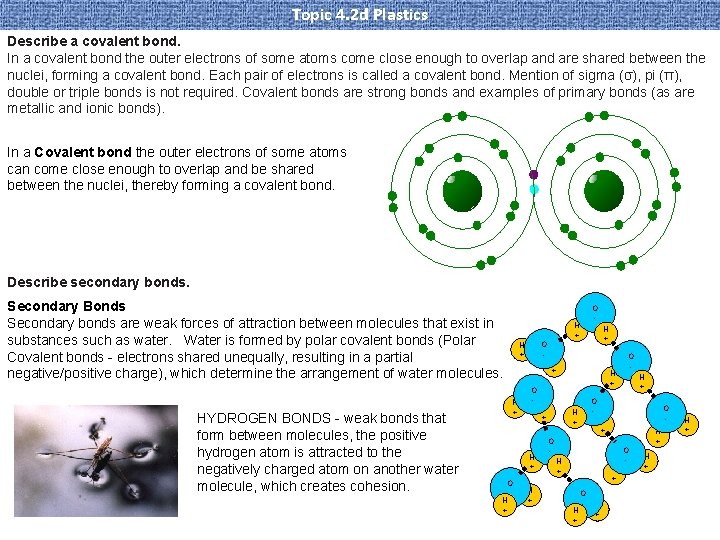
Topic 4. 2 d Plastics Describe a covalent bond. In a covalent bond the outer electrons of some atoms come close enough to overlap and are shared between the nuclei, forming a covalent bond. Each pair of electrons is called a covalent bond. Mention of sigma (σ), pi (π), double or triple bonds is not required. Covalent bonds are strong bonds and examples of primary bonds (as are metallic and ionic bonds). In a Covalent bond the outer electrons of some atoms can come close enough to overlap and be shared between the nuclei, thereby forming a covalent bond. Describe secondary bonds. Secondary Bonds Secondary bonds are weak forces of attraction between molecules that exist in substances such as water. Water is formed by polar covalent bonds (Polar Covalent bonds - electrons shared unequally, resulting in a partial negative/positive charge), which determine the arrangement of water molecules. HYDROGEN BONDS - weak bonds that form between molecules, the positive hydrogen atom is attracted to the negatively charged atom on another water molecule, which creates cohesion. O - H + H + O - H + O H + H + H + O - O - H + H +

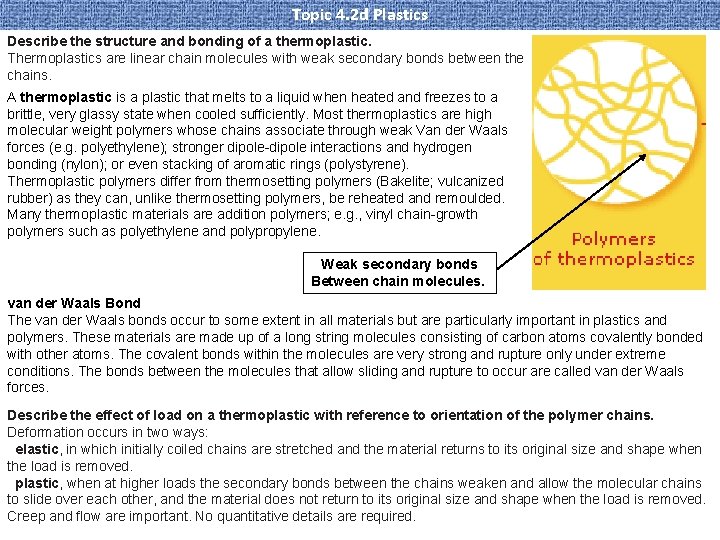
Topic 4. 2 d Plastics Describe the structure and bonding of a thermoplastic. Thermoplastics are linear chain molecules with weak secondary bonds between the chains. A thermoplastic is a plastic that melts to a liquid when heated and freezes to a brittle, very glassy state when cooled sufficiently. Most thermoplastics are high molecular weight polymers whose chains associate through weak Van der Waals forces (e. g. polyethylene); stronger dipole-dipole interactions and hydrogen bonding (nylon); or even stacking of aromatic rings (polystyrene). Thermoplastic polymers differ from thermosetting polymers (Bakelite; vulcanized rubber) as they can, unlike thermosetting polymers, be reheated and remoulded. Many thermoplastic materials are addition polymers; e. g. , vinyl chain-growth polymers such as polyethylene and polypropylene. Weak secondary bonds Between chain molecules. van der Waals Bond The van der Waals bonds occur to some extent in all materials but are particularly important in plastics and polymers. These materials are made up of a long string molecules consisting of carbon atoms covalently bonded with other atoms. The covalent bonds within the molecules are very strong and rupture only under extreme conditions. The bonds between the molecules that allow sliding and rupture to occur are called van der Waals forces. Describe the effect of load on a thermoplastic with reference to orientation of the polymer chains. Deformation occurs in two ways: elastic, in which initially coiled chains are stretched and the material returns to its original size and shape when the load is removed. plastic, when at higher loads the secondary bonds between the chains weaken and allow the molecular chains to slide over each other, and the material does not return to its original size and shape when the load is removed. Creep and flow are important. No quantitative details are required.

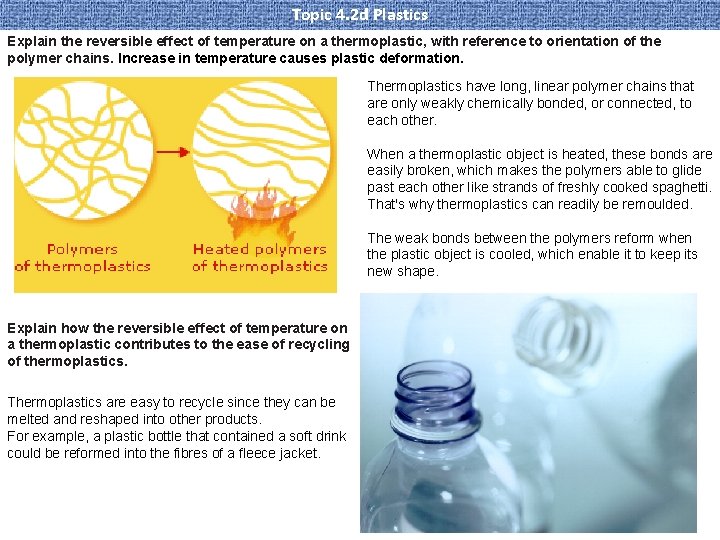
Topic 4. 2 d Plastics Explain the reversible effect of temperature on a thermoplastic, with reference to orientation of the polymer chains. Increase in temperature causes plastic deformation. Thermoplastics have long, linear polymer chains that are only weakly chemically bonded, or connected, to each other. When a thermoplastic object is heated, these bonds are easily broken, which makes the polymers able to glide past each other like strands of freshly cooked spaghetti. That's why thermoplastics can readily be remoulded. The weak bonds between the polymers reform when the plastic object is cooled, which enable it to keep its new shape. Explain how the reversible effect of temperature on a thermoplastic contributes to the ease of recycling of thermoplastics. Thermoplastics are easy to recycle since they can be melted and reshaped into other products. For example, a plastic bottle that contained a soft drink could be reformed into the fibres of a fleece jacket.

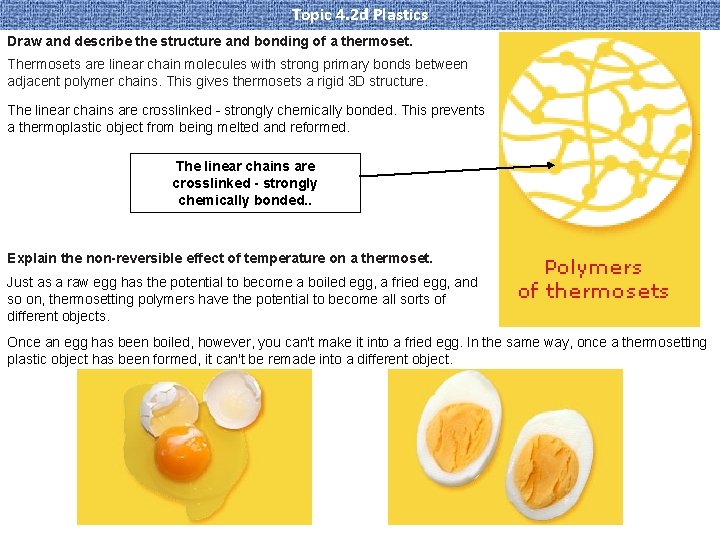
Topic 4. 2 d Plastics Draw and describe the structure and bonding of a thermoset. Thermosets are linear chain molecules with strong primary bonds between adjacent polymer chains. This gives thermosets a rigid 3 D structure. The linear chains are crosslinked - strongly chemically bonded. This prevents a thermoplastic object from being melted and reformed. The linear chains are crosslinked - strongly chemically bonded. . Explain the non-reversible effect of temperature on a thermoset. Just as a raw egg has the potential to become a boiled egg, a fried egg, and so on, thermosetting polymers have the potential to become all sorts of different objects. Once an egg has been boiled, however, you can't make it into a fried egg. In the same way, once a thermosetting plastic object has been formed, it can't be remade into a different object.

Topic 4. 2 d Plastics Discuss the properties and uses of polypropene and polyethene thermoplastic materials. Polypropylene or polypropene (PP) is a thermoplastic polymer, made by the chemical industry and used in a wide variety of applications, including packaging, textiles (e. g. , ropes, thermal underwear and carpets), stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes. Common extrusion methods include production of melt blown and spun bond fibres to form long rolls for future conversion into a wide range of useful products such as face masks, filters, diapers and wipes. The most common shaping technique is injection moulding, which is used for parts such as cups, cutlery, vials, caps, containers, housewares and automotive parts such as batteries. The related techniques of blow moulding and are also used, which involve both extrusion and moulding. The large number of end use applications for PP are often possible because of the ability to tailor grades with specific molecular properties and additives during its manufacture. For example, can be added to help PP surfaces resist dust and dirt. Many physical finishing techniques can also be used on PP, such as machining. Surface treatments can be applied to PP parts in order to promote adhesion of printing ink and paints. http: //en. wikipedia. org/wiki/Polypropene Polypropylene lid of a Tic Tacs box, with a living hinge and the resin identification code under its flap

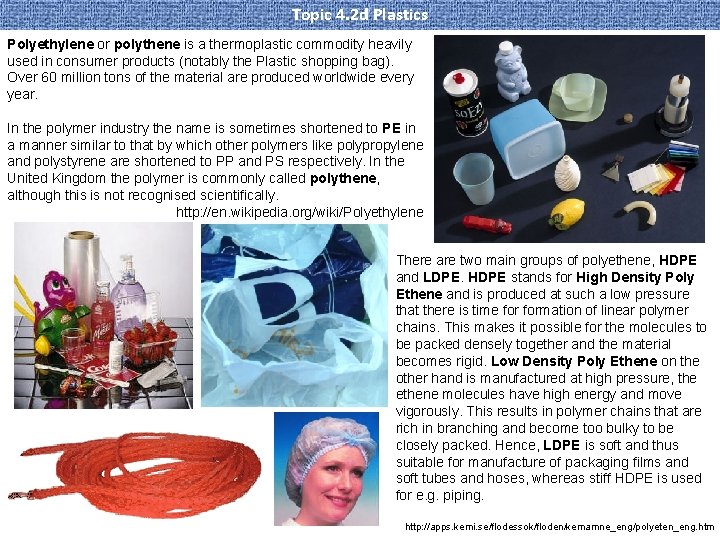
Topic 4. 2 d Plastics Polyethylene or polythene is a thermoplastic commodity heavily used in consumer products (notably the Plastic shopping bag). Over 60 million tons of the material are produced worldwide every year. In the polymer industry the name is sometimes shortened to PE in a manner similar to that by which other polymers like polypropylene and polystyrene are shortened to PP and PS respectively. In the United Kingdom the polymer is commonly called polythene, although this is not recognised scientifically. http: //en. wikipedia. org/wiki/Polyethylene There are two main groups of polyethene, HDPE and LDPE. HDPE stands for High Density Poly Ethene and is produced at such a low pressure that there is time formation of linear polymer chains. This makes it possible for the molecules to be packed densely together and the material becomes rigid. Low Density Poly Ethene on the other hand is manufactured at high pressure, the ethene molecules have high energy and move vigorously. This results in polymer chains that are rich in branching and become too bulky to be closely packed. Hence, LDPE is soft and thus suitable for manufacture of packaging films and soft tubes and hoses, whereas stiff HDPE is used for e. g. piping. http: //apps. kemi. se/flodessok/floden/kemamne_eng/polyeten_eng. htm

Topic 4. 2 d Plastics Discuss the properties and uses of polyurethane and urea–formaldehyde (methanal) thermoset materials. Polyurethane, commonly abbreviated PU, is any polymer consisting of a chain of organic units joined by urethane links. Polyurethane formulations cover an extremely wide range of stiffness, hardness, and densities. These materials include: q low density flexible foam used in upholstery and bedding, q low density rigid foam used for thermal insulation and e. g. automobile dashboards, qsoft solid elastomers used for gel pads and print rollers, q hard solid plastics used as electronic instrument bezels and structural parts. http: //en. wikipedia. org/wiki/Polyurethane flexible polyurethane foam

Topic 4. 2 d Plastics Urea-formaldehyde, also known as urea-methanal, named so for its common synthesis pathway and overall structure, is a transparent thermosetting resin or plastic, made from urea and formaldehyde heated in the presence of a mild base such as ammonia or pyridine. These resins are used in adhesives, finishes, MDF, and moulded objects. Urea-formaldehyde resin's attributes include high tensile strength, flexural modulus and heat distortion temperature, low water absorption, mould, high surface hardness, elongation at break, and volume resistance. Urea formaldehyde was commonly used when producing electrical appliances casing i. e. desk lamps. It is now mostly replaced by melamine resin. Urea-formaldehyde foam insulation started being used in the 1950 s. In the 1980 s, concerns began to develop about the toxic formaldehyde vapour emitted in the curing process, as well as from the breakdown of old foam. Consequently, its use was discontinued. http: //en. wikipedia. org/wiki/Urea-Formaldehyde

Topic 4. 2 d Plastics Discuss the issues associated with the disposal of plastics, for example, polyvinyl chloride (PVC). Although PVC disposal is problematic, PVC is still widely used as a structural material, for example, in windows and for guttering and drainpipes. http: //www. foe. co. uk/resource/factsheets/plastics. pdf Plastic facts in the UK: q We produce 3 million tonnes of plastic every year; q Households are the biggest producers of plastic waste; q 60% of household waste comes from packaging; q Over 60% of litter on beaches is plastic; q More than 80% of all this plastic is used once and then thrown into landfill sites; q Only 7% of plastic is recycled. Plastics and the environment There are environmental impacts from plastic production, plastic use and plastic waste disposal. Plastic containers are now lighter than they used to be, using less material, but our consumption of plastic is still set to rise. The building blocks of plastic, known as monomers, are made from oil and gas (plastic production uses 8% of the world oil production each year). To make the various polymers used by industry for various uses (for example, PET for plastic bottles, polystyrene, PVC) many chemicals are used which have not undergone a risk assessment. Plastic disposal problems Incineration: Energy can be obtained from burning waste, but this is less energy than can be saved by recycling. In addition: q Incineration destroys valuable resources; q Burning fossil fuels like coal, oil and gas is causing increasing levels of carbon dioxide in our atmosphere, leading to climate change. Incineration contributes to climate change, because when materials are burnt more fossil fuel energy is used to replace them through mining, manufacturing, and transportation around the world. Energy from burning waste is not renewable; q Incinerators need a steady stream of waste to keep them going. This means there is no incentive to reduce waste or recycle it; q Incineration causes pollution from air emissions and toxic ash; q Incineration is worse for climate change than recycling because new products have to be made to qreplace those destroyed; q Incineration does not provide thousands of new job opportunities that recycling does.

Topic 4. 2 d Plastics Plastic disposal problems Landfill: Friends of the Earth wants a ban on new landfill capacity until policies are in place to achieve the 60 80 per cent recycling rates achieved in other countries, because: q Landfill wastes valuable resources; q Landfill contributes to climate change, because when materials are buried more fossil fuel energy is used to replace them through mining, manufacturing, and transportation around the world; q Landfill produces methane, a powerful greenhouse gas which contributes to climate change; q Landfill creates water pollution as liquid from landfill sites leaks into our water supply; q Landfill can lead to land contamination; q Landfill leads to increased traffic, noise, smell, smoke, dust and litter. http: //globalplasticrecyclers. com/files/DSC 05104 -300 x 225. JPG http: //www. besafenet. com/PVC 04/PVCExe. Summary. htm

Topic 4. 2 d Plastics The PVC waste crisis (from Greenpeace http: //archive. greenpeace. org/toxics/html/content/pvc 3. html#recycle) The world is facing a waste crisis from PVC. Short-life PVC products, disposed of within a few years, have caused serious PVC waste problems, especially when incinerated. The average life span of the durable products -which make up more than half of PVC consumption - is around 34 years. Durable vinyl goods produced and sold since the 1960 s - when the plastic boom began - are now just starting to enter the waste stream. We are only now seeing the first stages of an impending PVC waste mountain. There are currently over 150 million tonnes of long-life PVC materials in existence globally, used mostly in the construction sector, which will constitute this waste mountain in coming decades. Taking into account the ongoing growth in production, by the year 2005 this amount will double and the world will have to deal with approximately 300 million tonnes of PVC starting to enter the waste stream. The amount of PVC waste arising in industrialised countries is already expected to grow faster than PVC production. So what do we do with this waste? Is there a solution? Since PVC, like most plastics, does not biodegrade quickly, three primary options exist: bury it, incinerate it or recycle it. PVC Recycling In the late 1980 s, PVC recycling was promoted by the vinyl industry in order to make PVC more acceptable to the public and to prevent government action to limit PVC production and use. As a result, the general public and decision-makers are now accepting recycling as a technical solution to the environmental problems associated with PVC. This is especially the case in countries with advanced recycling policies, like Denmark, Germany, the Netherlands and the USA. In reality, Greenpeace has found that PVC recycling in the main PVC consuming regions of the world amounts to less then one per cent of consumption. According to independent research, for 70 - 85% of PVC waste, recycling is not even an option for the mid to long term. This means hundreds of thousands of tonnes of PVC waste are destined to become waste in the near future creating a growing disposal problem.

Topic 4. 2 d Plastics PVC and Incineration is not a sustainable option for dealing with waste. When plastic is burned, less energy is generated from it than was used to make it, and incineration also means that the carbon contained within it is emitted as CO 2 - a greenhouse gas. Toxic substances are also emitted, and large amounts of solid wastes are produced as slag, ash, filter residues and neutralisation salt residues. When chlorine-containing materials, such as PVC, are burned dioxins are created. The higher the chlorine content of the materials burned, the greater the quantity of dioxins formed. In many countries, PVC is the single largest chlorine source in municipal waste; a significant amount of research has shown an association between chlorine input and dioxin output in hospital and municipal garbage incinerators. Incineration of PVC is not just a problem because of dioxin emissions. Burning PVC also produces at least 75 byproducts of combustion, including carcinogens such as vinyl chloride, PCBs, chlorobenzene and other aromatic hydrocarbons such as benzene, toluene, xylene, and naphthalene. Toxic ingredients added to PVC to give it useful properties -- such as lead, cadmium, and phthalates -- are also released during incineration. These will be emitted to the air or in the ash that will be landfilled. Because huge quantities of heavy metals are added to PVC as stabilisers, PVC is the major source of lead and cadmium in the municipal waste stream. In Germany, PVC incineration releases more lead into the environment than leaded gasoline and is considered the main source of cadmium emissions. Incinerating PVC increases the amount of hazardous waste that needs to be landfilled. The incineration of 1 kg PVC creates approximately 1 -3 kg of contaminated salt residues from the neutralisation of the hydrochloric acid when dry and semi dry neutralisation processes are applied. This salt needs to be disposed of in landfills as hazardous waste, making a mockery of the claim that incineration is a form of waste reduction.

Topic 4. 2 d Plastics PVC and Landfills PVC's additives will eventually leach, posing a risk to groundwater. The bulk of petrochemical-based plastics - such as PVC, Polypropylene (PP) and polyethylene (PE) - are durable and have a long lifetime. After disposal, they do not decompose readily or quickly. Moreover, the use of many different chemical additives in some plastics results in their leaching out of landfills to contaminate soil and groundwater. This is especially true for PVC, which has the highest content of additives, most of which are hazardous to the environment. Considerable quantities of PVC are present in landfills, as a result of the disposal of municipal solid wastes (MSW), and construction and other wastes. Almost 1 million tonnes of PVC was landfilled in Europe as MSW in 1994. PVC waste from other sectors, such as agriculture, the car industry, construction and distribution is included will add considerably to this. Landfill fires become particularly toxic with PVC waste. Landfill fires are a common occurrence, with the potential to pyrolyse and combust PVC, leading to the release - either in smoke or as leachate - of a range of pollutants, including heavy metal additives and dioxins.

Topic 4. 2 d Plastics PVC waste exports PVC waste is exported from the USA, Europe and Australia to developing countries, often for recycling into lower quality products such as shoes and low quality pipes, or 'downcycling'. According to the Indonesian Environment Minister, up to 40% of the plastic waste imported into Indonesia is not recycled but directly disposed of, partly as hazardous waste. Downcycled products will eventually be dumped or burned since downcycling simply delays the inevitable need to dispose of PVC plastic waste. In the USA, exporting PVC scrap and waste seems to be a more important disposal route than recycling; the amount of PVC scrap and waste exported in the first half of 1996 was greater than all the post consumer PVC recycled in the whole of 1995. In 1996, at least 4, 000 tonnes of preconsumer PVC waste was exported from the Netherlands to the Philippines for recycling into low quality products (Greenpeace Netherlands 1997). However, the actual amount of PVC waste exported for downcycling in Asia may be higher. Recycling of post-consumer and imported plastic waste is a growing income-generating industry in newly industrialising countries. However the dangers of working with mixed plastics contaminated with unknown substances may not be recognised. These include fumes, dust and other emissions from the reprocessing equipment and the need for protective clothing may be ignored.