Topic 4 14 BONDING My Name is Bond

Topic 4 / 14: BONDING My Name is Bond. Chemical Bond
PART 3: Hybridization & Delocalization of Electrons

Hybridization p Hybridization: a modification of the localized electron model to account for the observation that atoms often seem to use special atomic orbitals in forming molecules. This is part of both IB and AP curricula.
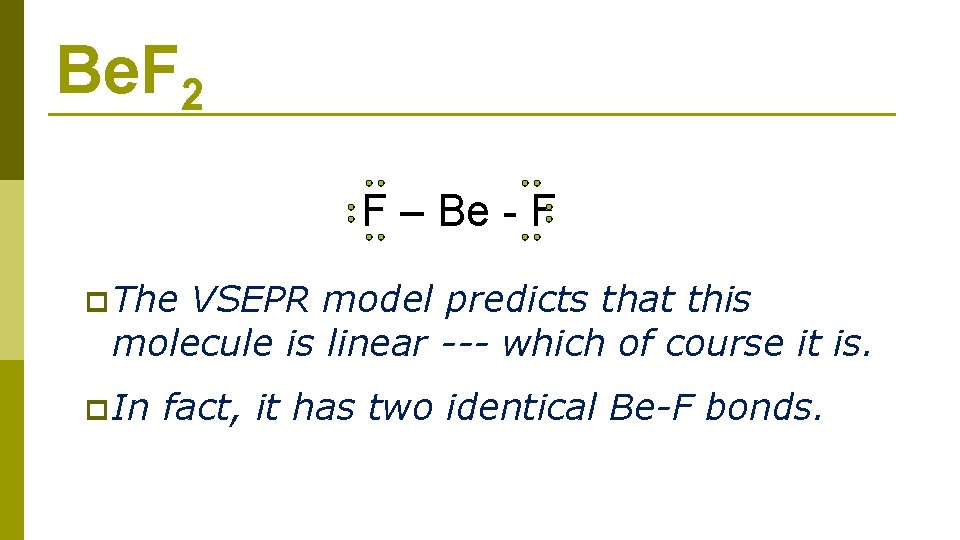
Be. F 2 F – Be - F p The VSEPR model predicts that this molecule is linear --- which of course it is. p In fact, it has two identical Be-F bonds.
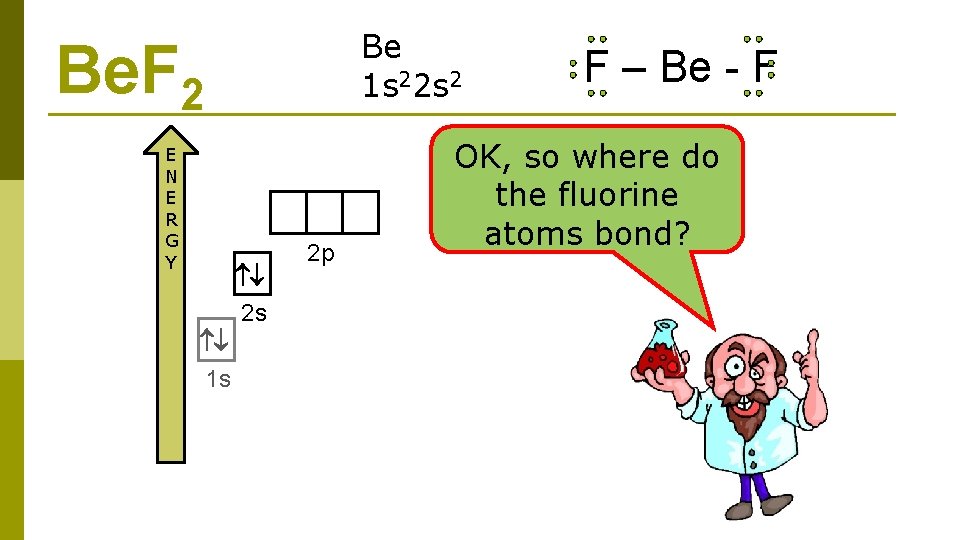
Be 1 s 22 s 2 Be. F 2 E N E R G Y 2 s 1 s 2 p F – Be - F OK, so where do the fluorine atoms bond?
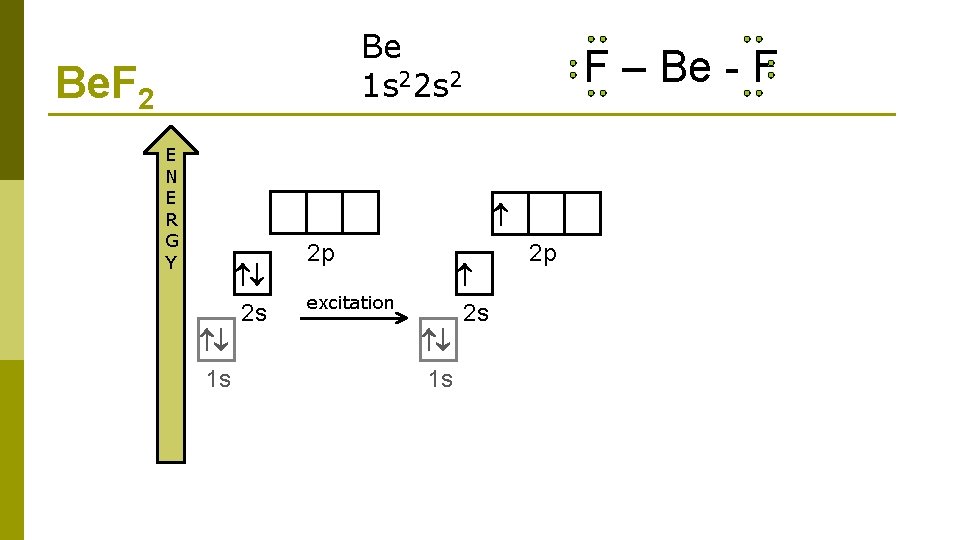
Be 1 s 22 s 2 Be. F 2 E N E R G Y F – Be - F 2 s 1 s 2 p 2 s excitation 1 s 2 p
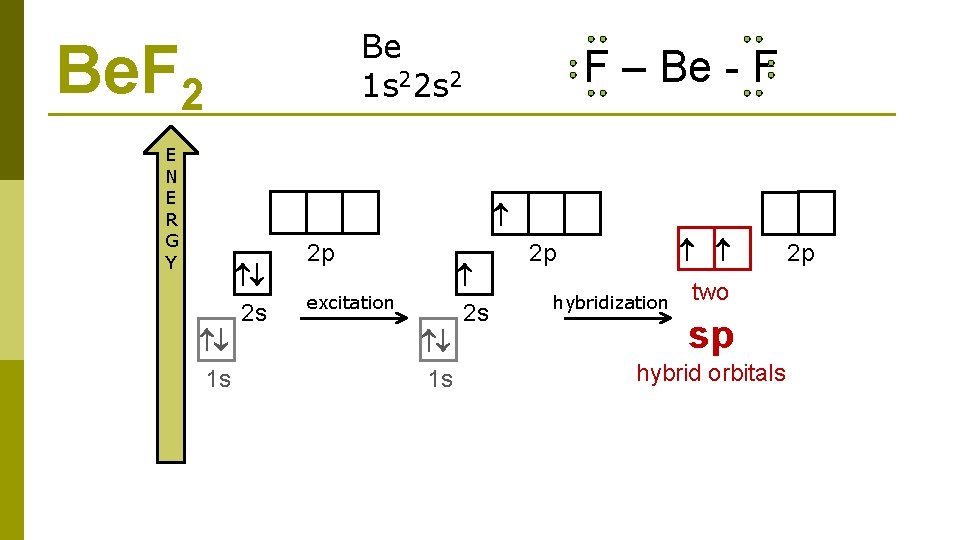
Be 1 s 22 s 2 Be. F 2 E N E R G Y F – Be - F 2 s 1 s 2 p 2 s excitation 1 s 2 p hybridization two sp hybrid orbitals 2 p
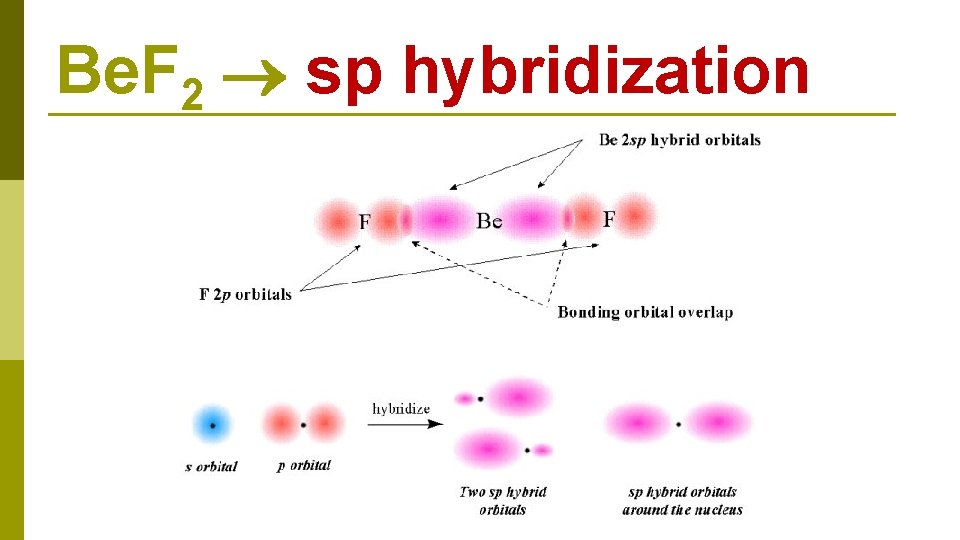
Be. F 2 sp hybridization
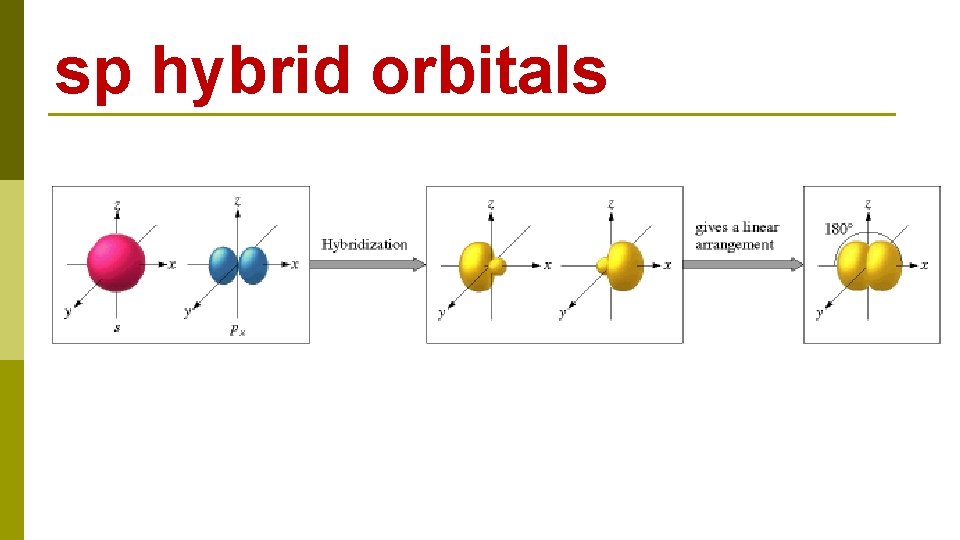
sp hybrid orbitals
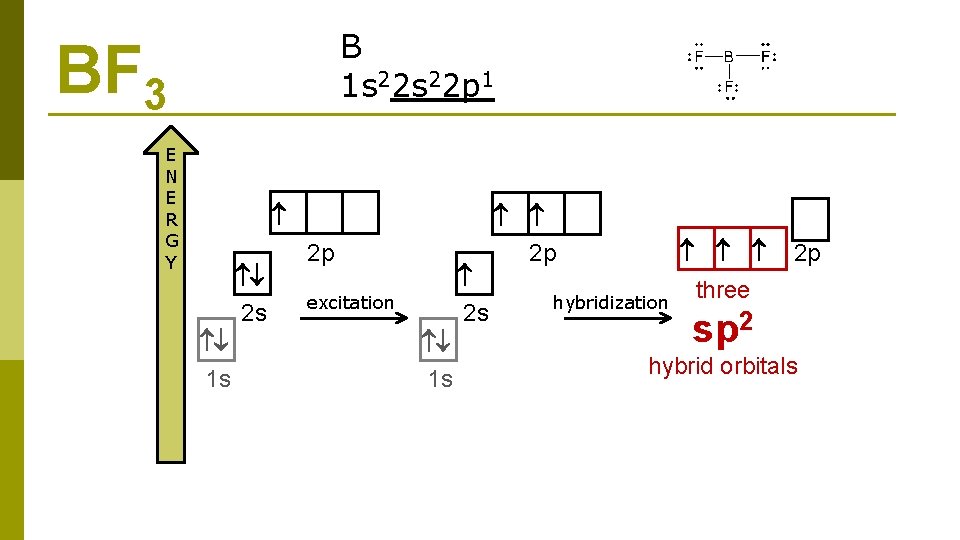
B 1 s 22 p 1 BF 3 E N E R G Y 2 s 1 s 2 p 2 s excitation 1 s 2 p hybridization 2 p three sp 2 hybrid orbitals
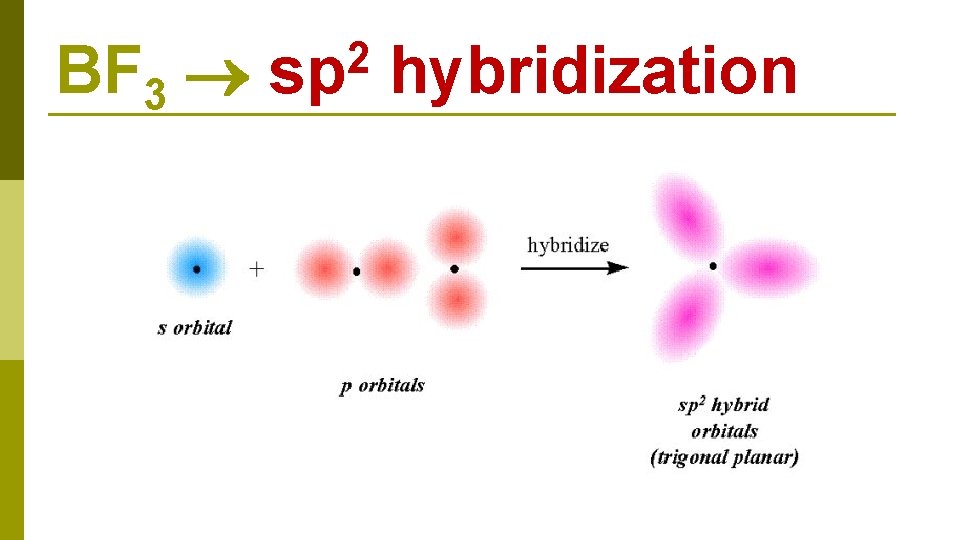
BF 3 2 sp hybridization
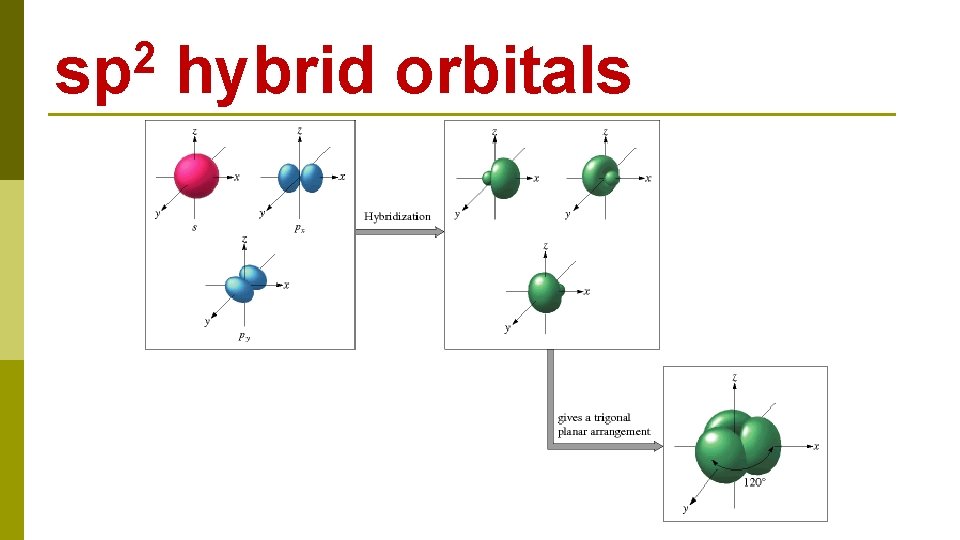
2 sp hybrid orbitals
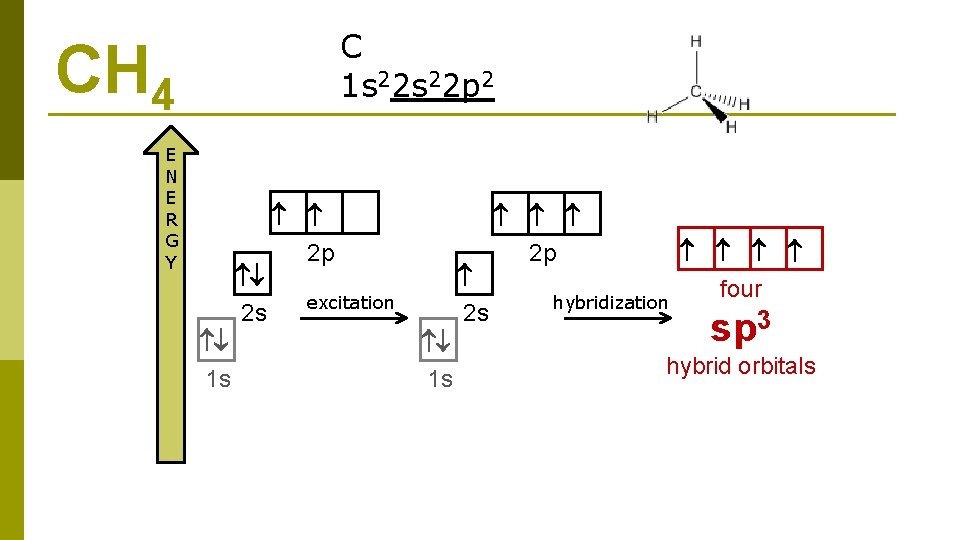
C 1 s 22 p 2 CH 4 E N E R G Y 2 s 1 s 2 p 2 s excitation 1 s 2 p hybridization four sp 3 hybrid orbitals
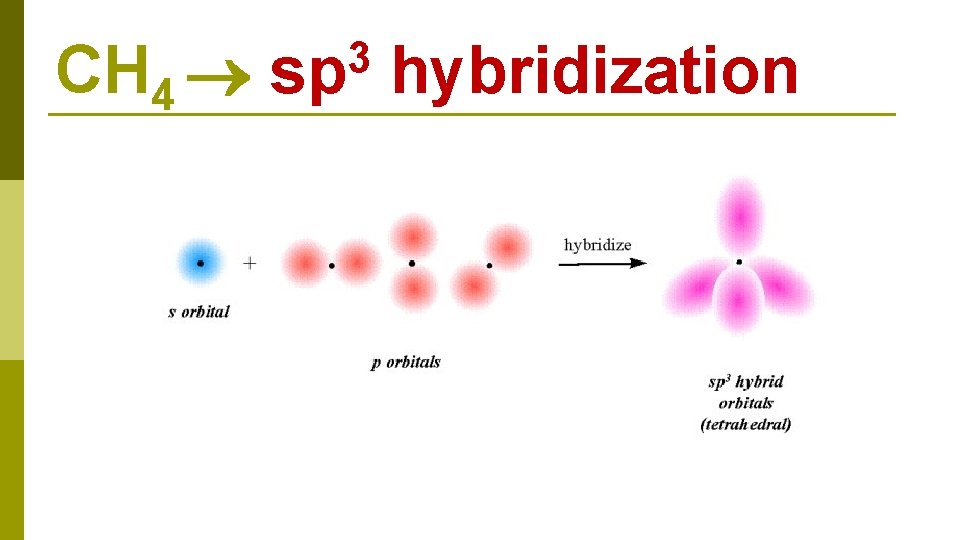
CH 4 3 sp hybridization
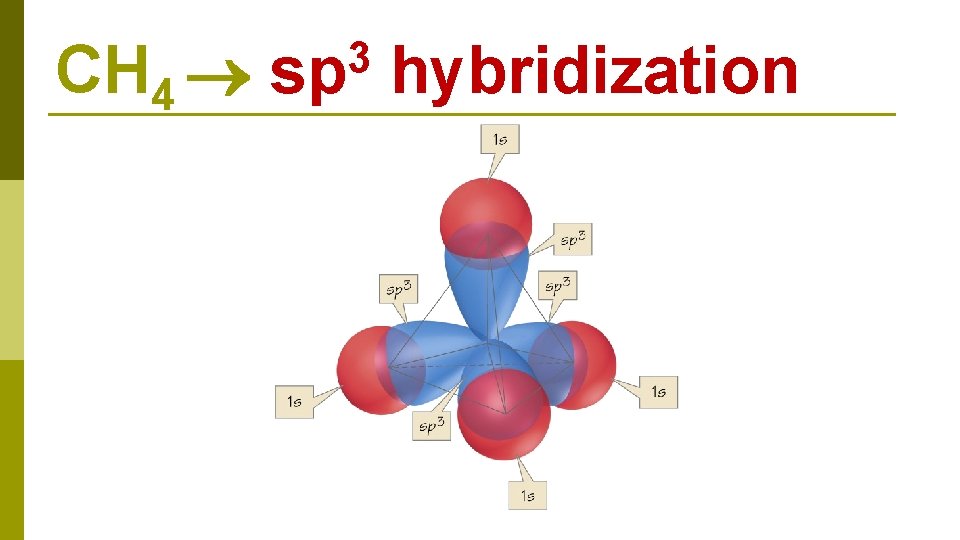
CH 4 3 sp hybridization
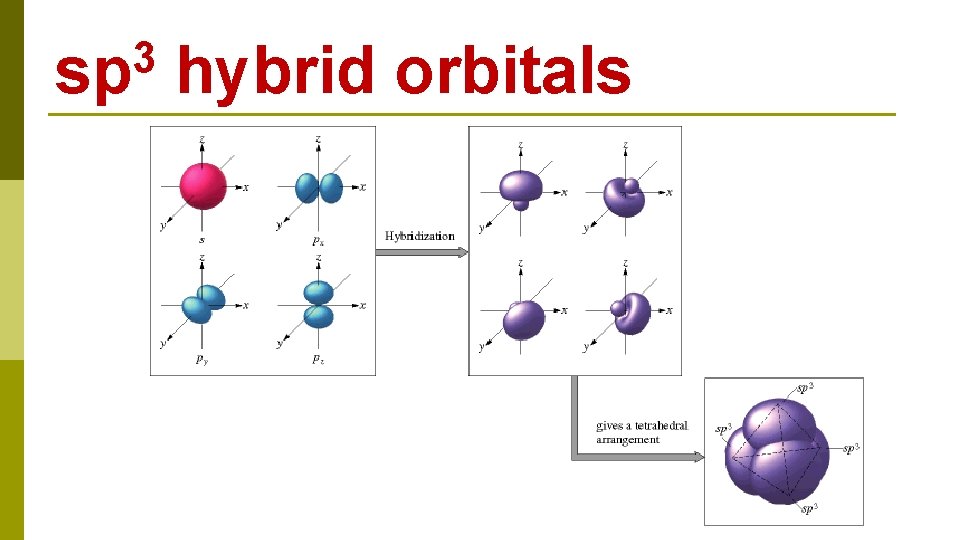
3 sp hybrid orbitals
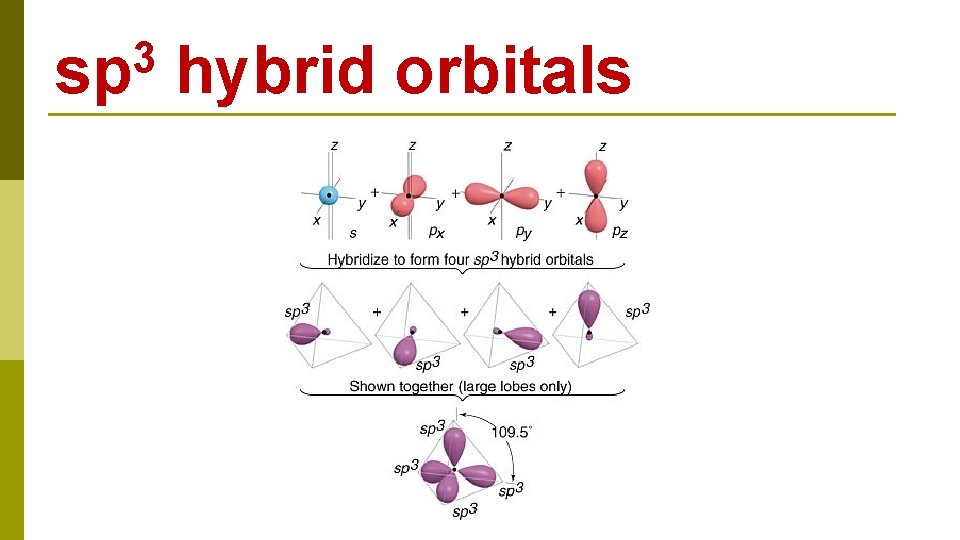
3 sp hybrid orbitals
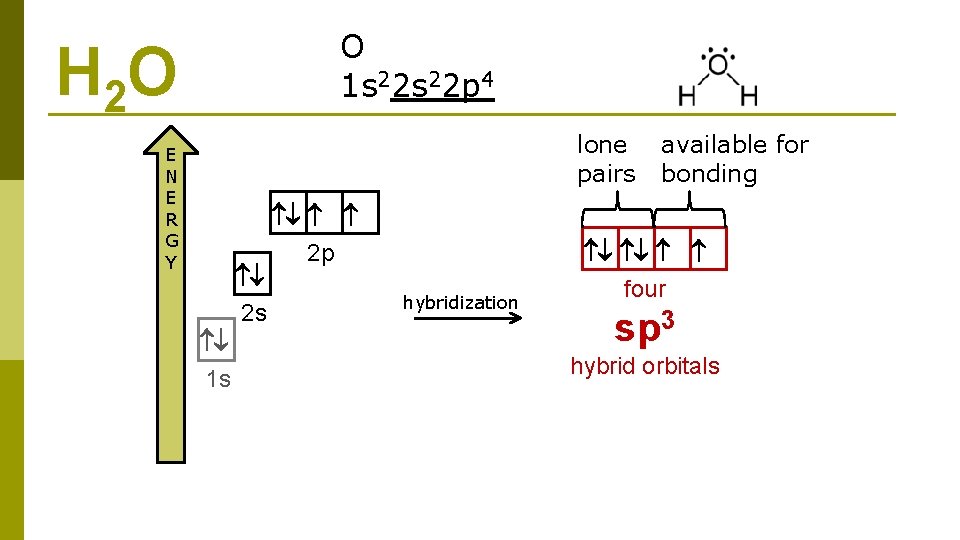
O 1 s 22 p 4 H 2 O lone pairs E N E R G Y 2 s 1 s 2 p hybridization available for bonding four sp 3 hybrid orbitals
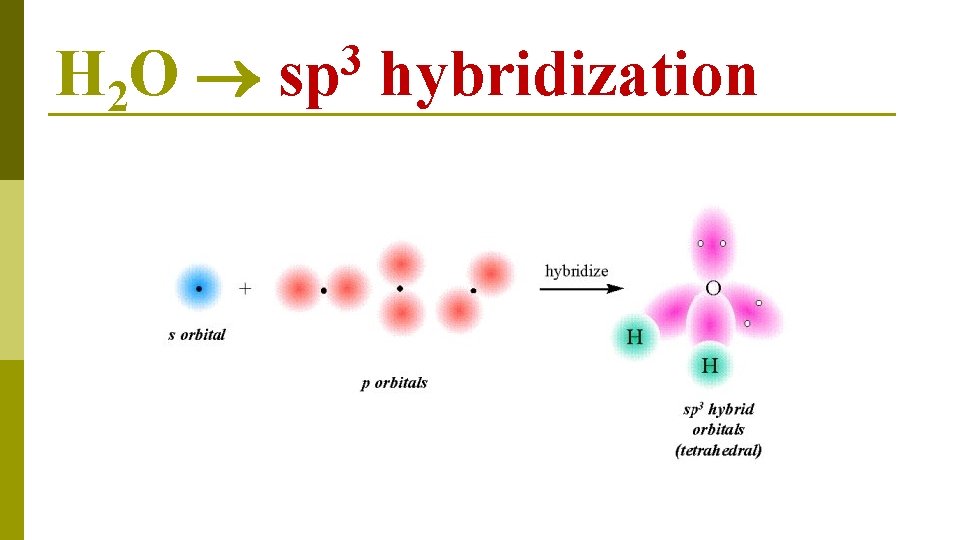
H 2 O 3 sp hybridization
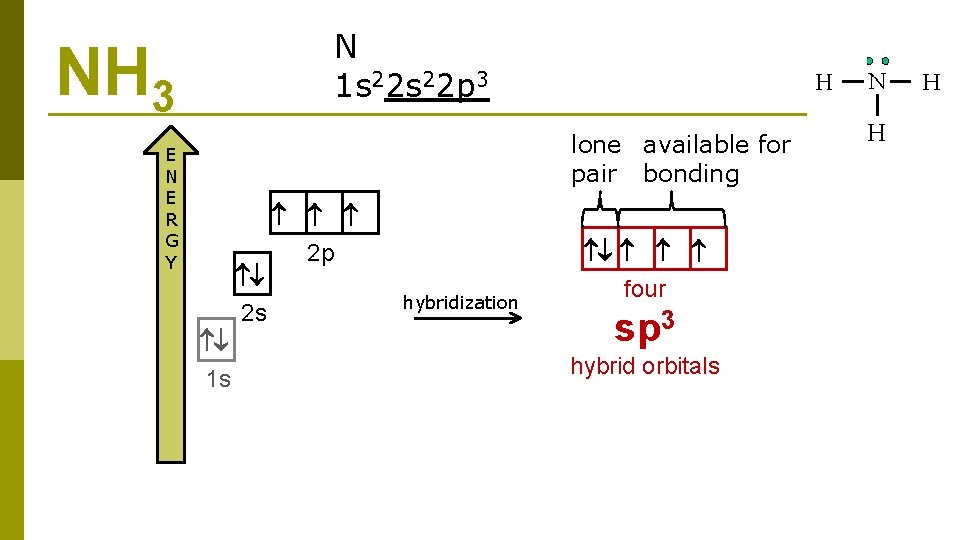
N 1 s 22 p 3 NH 3 lone available for pair bonding E N E R G Y 2 s 1 s 2 p hybridization four sp 3 hybrid orbitals
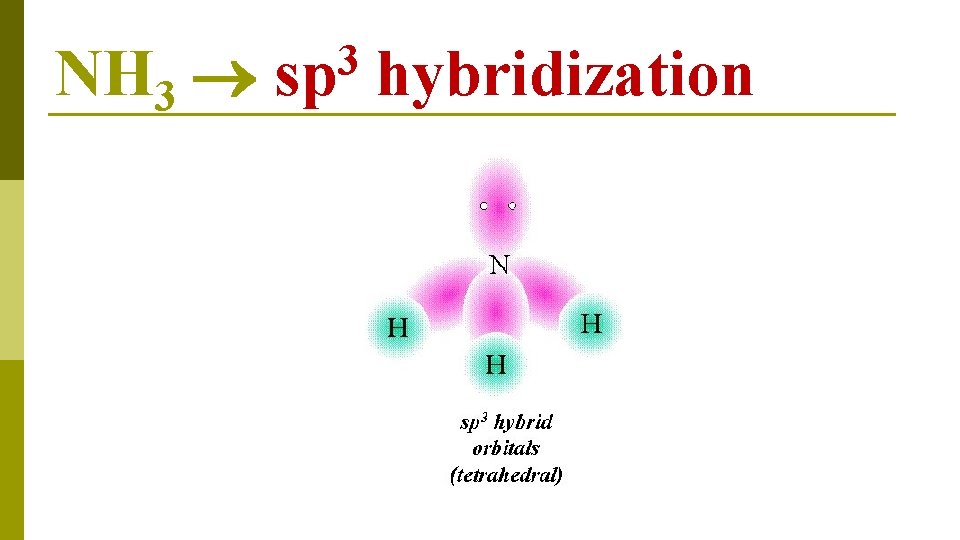
NH 3 3 sp hybridization
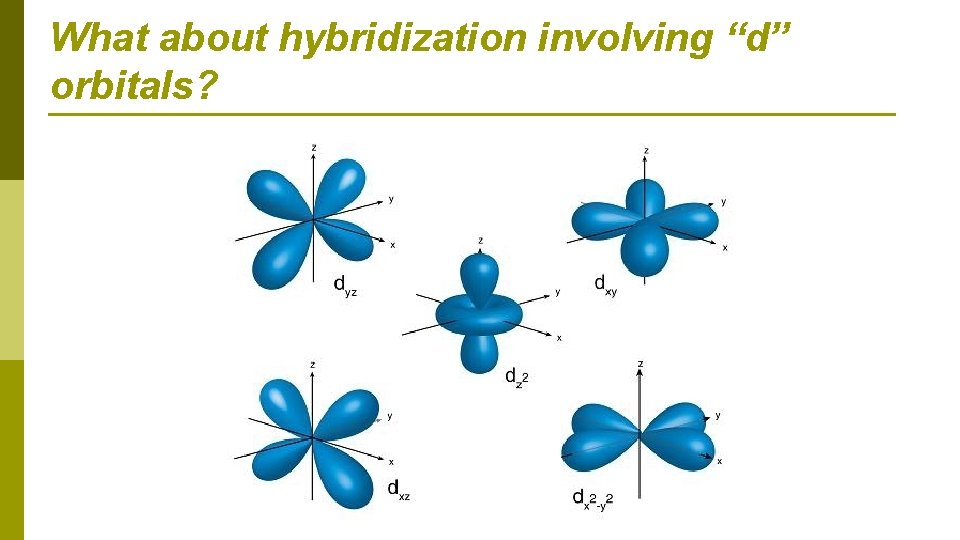
What about hybridization involving “d” orbitals?
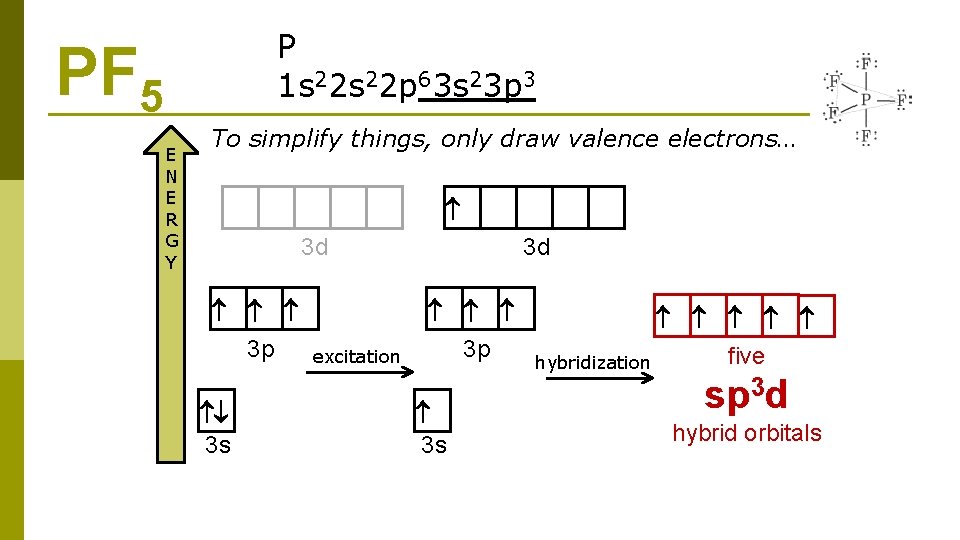
P 1 s 22 p 63 s 23 p 3 PF 5 E N E R G Y To simplify things, only draw valence electrons… 3 d 3 p 3 s excitation 3 d 3 p 3 s five hybridization sp 3 d hybrid orbitals
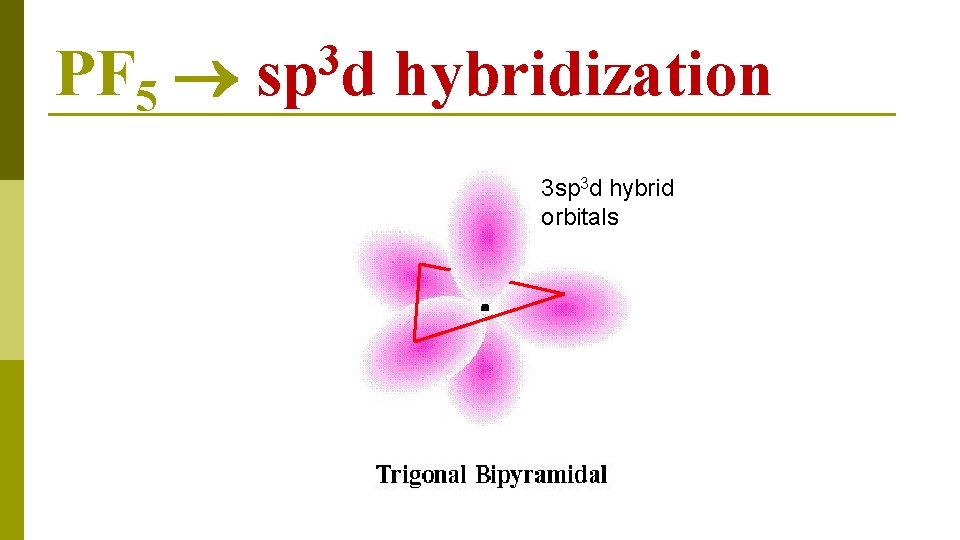
PF 5 3 sp d hybridization 3 sp 3 d hybrid orbitals
Something to think about: is hybridization a real process or simply a mathematical device (a human construction) we’ve concocted to explain how electrons interact when new chemical substances are formed?
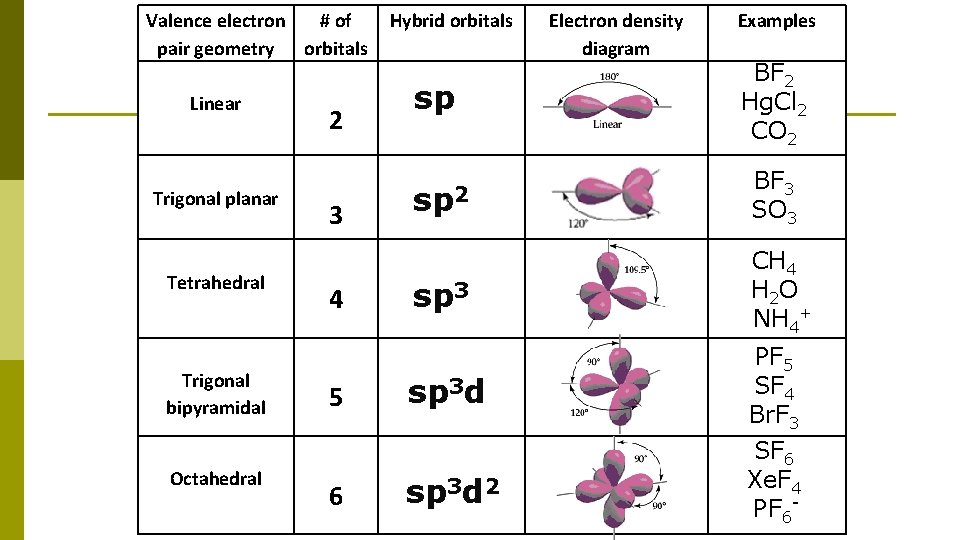
Valence electron # of pair geometry orbitals Linear Trigonal planar Tetrahedral Trigonal bipyramidal Octahedral 2 3 4 5 6 Hybrid orbitals Electron density diagram Examples sp BF 2 Hg. Cl 2 CO 2 sp 2 BF 3 SO 3 sp 3 CH 4 H 2 O NH 4+ sp 3 d PF 5 SF 4 Br. F 3 sp 3 d 2 SF 6 Xe. F 4 PF 6 -
and bonds p In Hybridization Theory there are two names for bonds, sigma ( ) and pi ( ). p Sigma bonds are the primary bonds used to covalently attach atoms to each other. p Pi bonds are used to provide the extra electrons needed to fulfill octet requirements.
and bonds p Every pair of bonded atoms shares one or more pairs of electrons. In every bond at least one pair of electrons is localized in the space between the atoms, in a sigma ( ) bond. p The electrons in a sigma bond are localized in the region between two bonded atoms and do not make a significant contribution to the bonding between any other atoms.
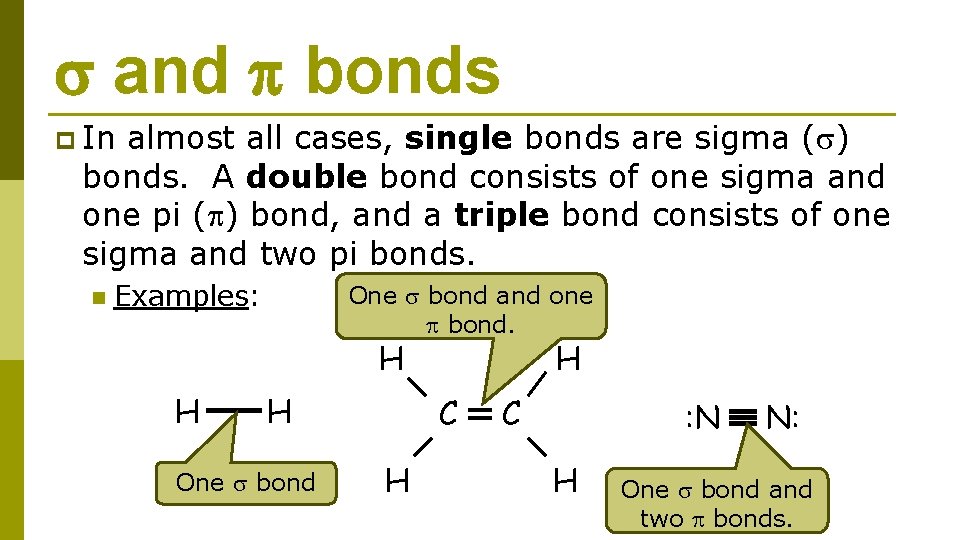
and bonds p In almost all cases, single bonds are sigma ( ) bonds. A double bond consists of one sigma and one pi ( ) bond, and a triple bond consists of one sigma and two pi bonds. n Examples: H H One bond and one bond. H H C C H : N H N: One bond and two bonds.
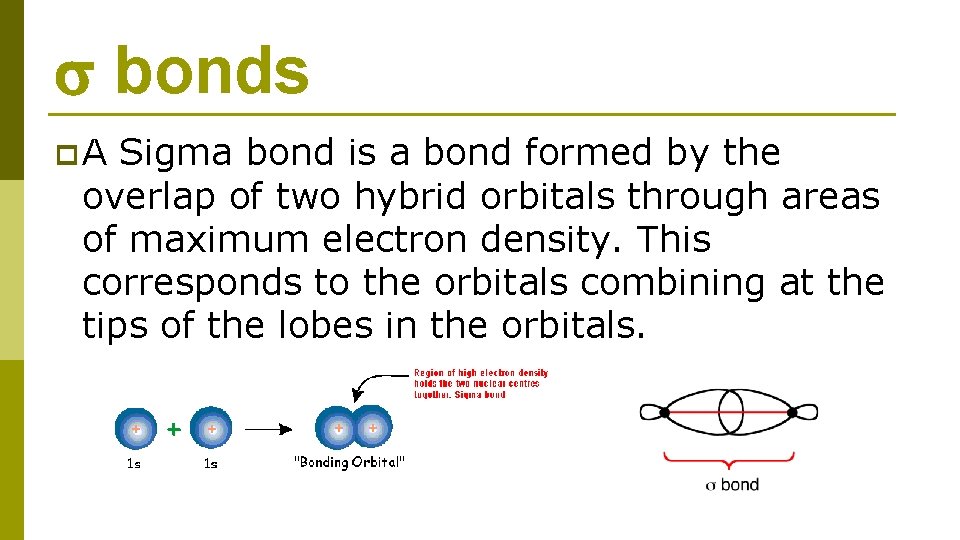
bonds p. A Sigma bond is a bond formed by the overlap of two hybrid orbitals through areas of maximum electron density. This corresponds to the orbitals combining at the tips of the lobes in the orbitals.
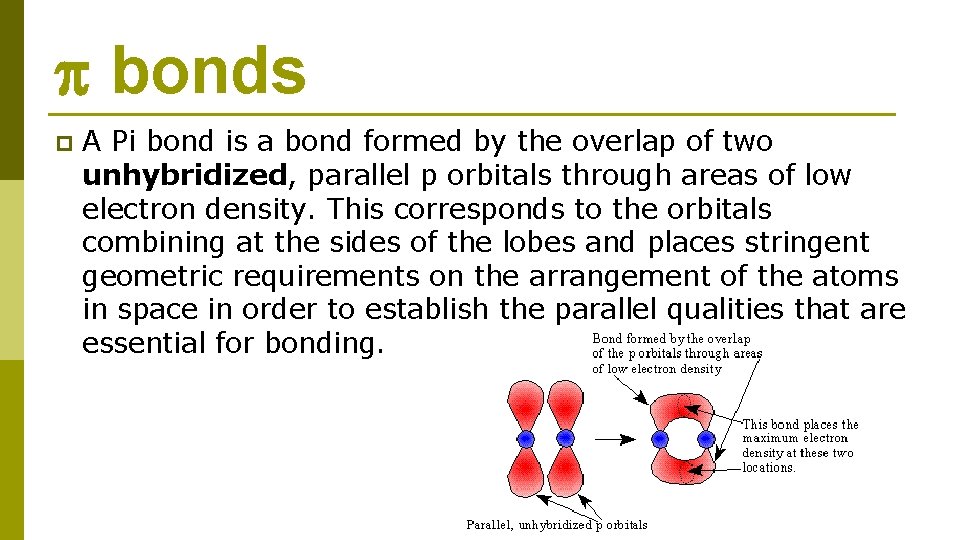
bonds p A Pi bond is a bond formed by the overlap of two unhybridized, parallel p orbitals through areas of low electron density. This corresponds to the orbitals combining at the sides of the lobes and places stringent geometric requirements on the arrangement of the atoms in space in order to establish the parallel qualities that are essential for bonding.

Remember – π bonds are unhybridized strawberry pie rhubarb pie strawberry-rhubarb pie

- Slides: 33