Topic 3 What are Elements What are Elements
















- Slides: 24

Topic 3 What are Elements?

What are Elements? �Meet the elements: https: //www. youtube. com/watch? v=d 0 z. ION 8 xjb. M � Greek philosophers observed that rock could be broken down into smaller and smaller pieces until it became powder. � They then wondered how many times you could continue to break the particles of powder down until they couldn’t be broken down any further.

What are Elements? �Democritus described the smallest particle as atomos, meaning “indivisible. ” � He believed that each type of material was made up of a different atomos. � These different particles gave materials their own unique set of properties. These could then be added to make new materials with their own properties.

Some history…. � The original ‘elements’ were earth, air, fire and water. � Ancient Greek philosophers thought matter was made out of these four ‘elements’. � They thought all things were made from these four elements with varying degrees of hotness, coldness, dryness and wetness.

From Alchemy �For the next 2000 years, alchemists, people who were part magician, part scientist, carried out experiments. �They believed that they should be able to turn any metal into gold and they were not interested in understanding the nature of matter. �They did, however, perform some of the first chemistry experiments.

What are Elements? �Chemistry was formed by trying to find elements and many scientists had lots of crazy ideas on trying to make gold. �Many thought they could turn lead or copper into gold and one guy even thought he could turn his urine into gold.

What are elements? �Well, we all know that didn’t happen…. . So how did scientists search for elements? ? ?

Elements vs. Compounds Ø An element is a pure substance made up of only one type of particle, or atom. Ø Each element has its own unique set of distinguishing properties and cannot be broken down into simpler substances by means of a chemical change.

Elements vs. Compounds �A compound is a pure substance made up of 2 or more elements chemically combined together. Compounds can be broken down into the elements that they are composed of.

I guess they first had to know what elements were…. . �The Law of Definite Composition Compounds are pure substances that contain two or more elements combined together in fixed (or definite) proportions What does this mean? ? ? ▪ Water is made of hydrogen and oxygen ▪ 11% hydrogen, 89% oxygen ▪ Hydrogen Peroxide is made of hydrogen and water ▪ 6% hydrogen and 94% oxygen

Electrolysis �Using a “voltaic pile” scientists could separate compounds. �Voltaic piles are still used today – we call them batteries.

Electrolysis �Early scientists used voltaic piles to pass electricity through water. �Breaking down compounds into their separate elements by shooting electric current through the compound is called electrolysis. �Electrolysis was used to isolate potassium, sodium, magnesium, calcium, strontium and barium.

CHEMISTRY’S MOST WANTED… #1 - DALTON Dalton thought of atoms as billiard balls. He drew pictures of water with equal numbers of smaller hydrogen and larger oxygen atoms.

Dalton’s Atomic Theory � All matter is made up of small particles called atoms. � Atoms cannot be created, destroyed, or divided into smaller particles. � All atoms of the same element are identical in mass and size. Atoms of one element are different in mass and size form the atoms of other elements. � Compounds are created when atoms of different elements link together in a definite proportion.

Dalton’s Definitions �Element – pure substance made up of one type of particle or atom. Each element has its own distinct properties and cannot be broken down by means of chemical change. �Compound – pure substances that are made up of two or more different elements chemically combined together. Compounds can be broken down into elements by chemical means.

CHEMISTRY’S MOST WANTED… #2 - THOMSON Thought of atoms as negatively charged electrons stuck to a positively charged mass “Raisin Buns” “Plum Pudding”

CHEMISTRY’S MOST WANTED… #3 - RUTHERFORD Thought the entire mass of an atom was in the center of the atom (called the atomic nucleus) He thought the rest of the atom was just empty space

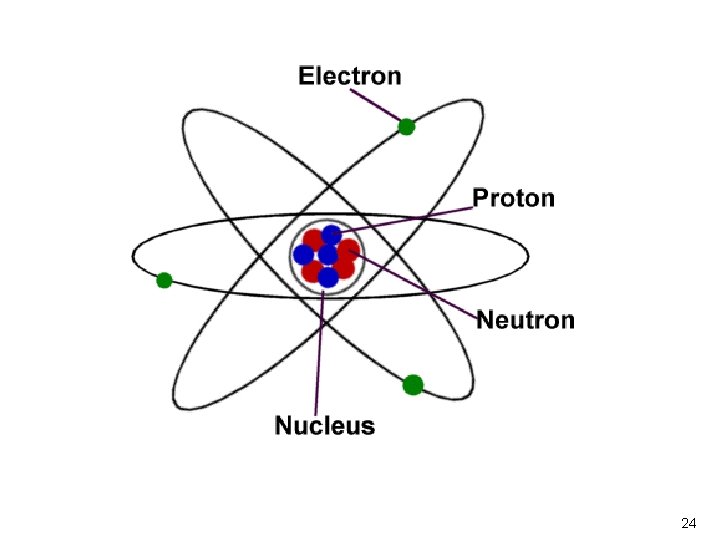
Rutherford’s Model �He called the positively charged particles in the center of the atom protons. �He called the negatively charged particles floating around the atomic nucleus, electrons.

CHEMISTRY’S MOST WANTED… #4 - BOHR Pictured the atom like a mini solar system. Electrons rotated around the nucleus like the planets around the sun in things called electron shells.

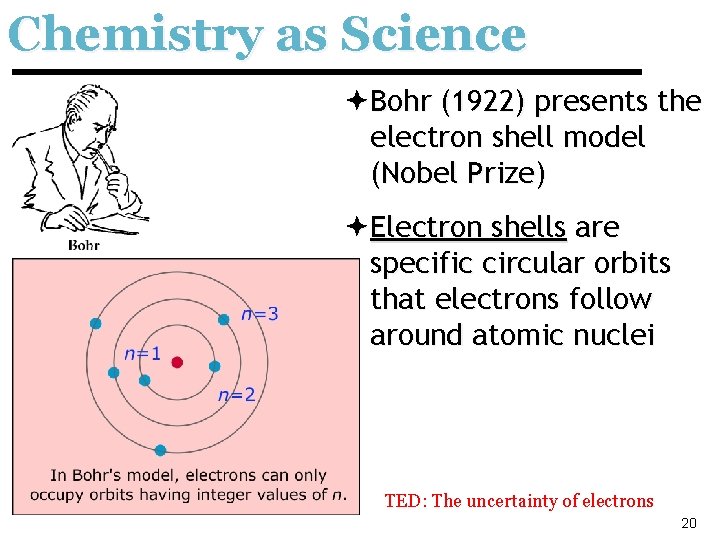
Chemistry as Science ªBohr (1922) presents the electron shell model (Nobel Prize) ªElectron shells are specific circular orbits that electrons follow around atomic nuclei TED: The uncertainty of electrons 20

Chadwick �Later on, Chadwick discovered Neutrons. He said these particles didn’t have a charge but helped make up the mass of the atom. Neutrons are in the atomic nucleus with the protons. http: //ed. ted. com/lessons/the-uncertain-location-of -electrons-george-zaidan-and-charles-morton

The story so far: - Matter has mass and takes up space Matter can be pure or a mixture Matter is made up of tiny particles called atoms Atoms cannot be broken down, created nor destroyed - Elements are made up of unique atoms. - Carbon atoms are different than Oxygen atoms 22

The Modern Atom ªChadwick discovers that the nucleus of an atom contains protons and neutrons (after 1922) ªNucleus is the positively charge center of an atom, containing protons and neutrons ªProtons are the positively charged particles in the atomic nucleus ªNeutrons are the neutrally charged particles in the atomic nucleus 23

24