Topic 3 Refrigerants 3 1 Primary and secondary

























- Slides: 28

Topic 3 Refrigerants 3. 1 Primary and secondary refrigerants 3. 2 Refrigerant selection criteria 3. 2. 1 Thermo-physical properties 3. 2. 2 Safety properties 3. 2. 3 Environmental properties 3. 2. 4 Economic properties 3. 3 Designation of refrigerants 3. 3. 1 Fully saturated, halogenated compounds 3. 3. 2 Inorganic refrigerants 3. 3. 3 Azeotropic and Zeotropic Mixtures 3. 3. 4 Hydrocarbons 3. 4 Comparison between different refrigerants 1

Refrigerants = cooling medium by extracting heat from space/things Vapor compression : evaporate and condense -100 C to +100 C at reasonable pressure The thermodynamic efficiency of a refrigeration system depends mainly on its operating temperatures However, other important practical issues System design Size Initial and operating costs Safety Reliability Serviceability type of refrigerant selected for a given application. 2

3. 1 Primary and secondary refrigerants Primary refrigerants – used in vapor-compression systems ice/dry ice (solid CO 2) – not primary refrigerants Two substances form refrigerant combination in absorption refrigeration system. refrigerant : butane, carbon tetrachloride, ethane, hexane, methane, pentane, propane, ammonia, methyl chloride, CO 2, SO 2, water, air, chloroform, Freon Secondary refrigerants – liquids used for transporting low-temp. heat energy from one location to another - antifreezes, brines, water Carry heat from cooled substance to evaporator Change in temperature Not change phase Water Brines Antifreezes Solutions with tfreezing < 0 C Solutions of water and ethylene glycol, propylene glycol, calcium chloride Safe in contact with food 3

Phase diagram of an antifreeze @t. A – liquid @concentration M Cooling from A to B – liquid Cooling antifreeze to C – liquid + ice % ice = 100* x 2 / (x 1 + x 2) % liquid = 100* x 1 / (x 1 + x 2) Cooling below D – solidifies the entire mixture E – Eutectic point: lowest temp. no solidification 4

Secondary refrigerants Convert a manufacturer’s data for water into data for an antifreeze Pressure drop in straight tubes: Liquid in tubes h = Nu k/D Nu = 0. 023 Re 0. 8 Pr 0. 4 Antifreezes of high concentration Re = VD / Pr = cp /k -- high viscosity for HT, low for friction pdrop -- low thermal conductivity -- low specific heat h & p Good operation: no more concentration than necessary to prevent its freezing 5

3. 2 Refrigerant selection criteria 3. 2. 1 Thermo-physical properties 1. Latent heat of vaporization hfg: large high R. E. , low mass/qe Clasuis eq. : as hfg pr trade-off: hfg & pr 2. Critical point Tcr : high to get optimum peak on p-h diagram as Tcr small superheat and flash COP trade-off: COP & Vol Low vapor pressure volumetric capacity 3. Freezing point: low to avoid frozen action, work at very low temp 5. Thermal conductivity: higher heat transfer coefficient 5. Viscosity: smaller frictional pressure drops 6. Liquid specific heat cpl: small large degree of subcooling, smaller flash 7. Vapor specific heat cpv: large small degree of superheat 6

Selection of refrigerants in comparison 1. Positive Pe: R 11: Pressure < patm (101 k. Pa)-- air leak in need purger R 22, R 502, R 717 : heavy vessel & pipe for high pressure 2. Low Pc: 3. Small pr (pc/pe) : for high vol and low power for compressor 4. Refrigerating effect (h 4 – h 1) considered with Wcompressor shown in COP R 717 – high R. E. but also high Wcompressor same order COP 5. vsuction/k. W of Refrigeration density R 22, R 502, R 717 w*vsuction/k. W density R 11, requires high vsuction/k. W low density centrifugal compressor 6. High COP: R 11 but used only for centrifugal compressor [te, tc]= [-15, 30] C BP, C +23. 7 -29. 8 -40. 8 -33. 3 COPcarnot = 5. 74 Low- 7

7. Compressor discharge temperature Graph of tdischarge & tc Isentropic compression from sat. vapor at 0 C With High tdischarge Ammonia compressors -- equipped with water-cooled heads High tdischarge oil breakdown Excessive wear Reduce life of valves Pressure ratio tdischarge 8

3. 2. 2 Safety properties 1. Toxicity suffocation : degree of concentration and time of exposure degree of hazard depends on: - Amount of refrigerant used vs total space - Type of occupancy - Presence of open flames - Odor of refrigerant, and - Maintenance condition *depends on the specific application 2. Flammability ASHRAE : six safety groups (A 1 to A 3 and B 1 to B 3) least hazardous A 1 (R 11, R 12, R 22, R 134 a, R 744, R 718) most hazardous B 3 (R 1140) *special precautions should be taken to avoid accidents R 717 - Flammable in 16 -25%vol in air, high toxic - Injurious or lethal in 0. 5 -1% for exposure of < 30 min R 12 - Nontoxic up to 20%vol in air for exposure of < 2 h R 11 R 22 - Slightly more toxic than R 12 R 502 9

3. Leak in refrigeration system Leak: refrigerants may contact with food product Halocarbons No effect on foods, furs, fabrics for short exposures R 717 Prolonged exposures – food tasting/smelling Foods contain naturally ammonia 0. 01 -0. 1% *should be easy to detect the leaks 4. Reaction with material (piping, vessels, compressor) R 717 With water -- reacts with copper, brass, cuprous alloys Iron and steel used in ammonia systems Halocarbons reacts with only zinc, not Cu, Al, iron, steel With water – form acids with attack most metals attack with natural rubber: Synethetic material – gaskets, sealing 5. Dielectric strength high dielectric strength refrigerants for hermetic compressors systems 10

6. How refrigerants combines with lubricating oil No chemical reaction, but miscibility of oil and refrigerant In reciprocating and screw compressor: oil from compressor condenser evaporator heat-transfer effectiveness Oil separator: to remove/prevent oil from reaching evaporator oil separator placed in discharge-gas line: removes and returns oil to compressor R 12 Miscible Need high velocity in suction-line to carry oil back to comp. Most popularity R 22 Partially miscible R 717 Not miscible R 502 Supplant in popularity, by its lower w*vsuction/k. W of Qe Oil can be drained from ammonia evaporator Become popular lately, by its comparable w*vsuction/k. W of Qe , lower tdischarge but more miscible, 11

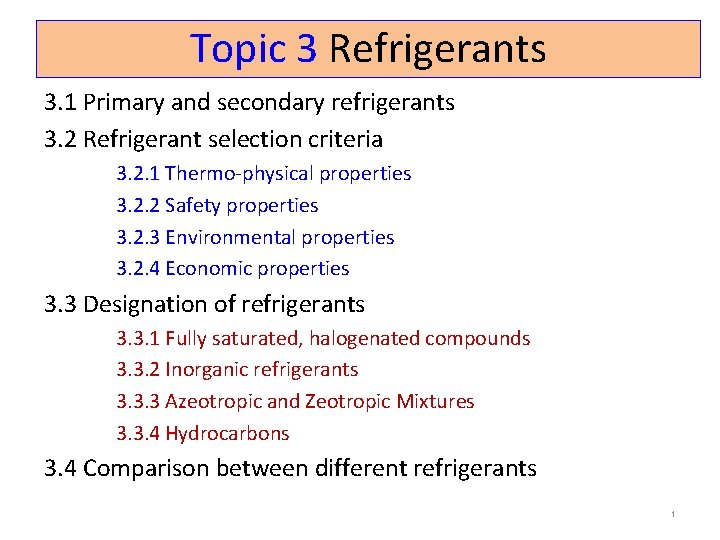
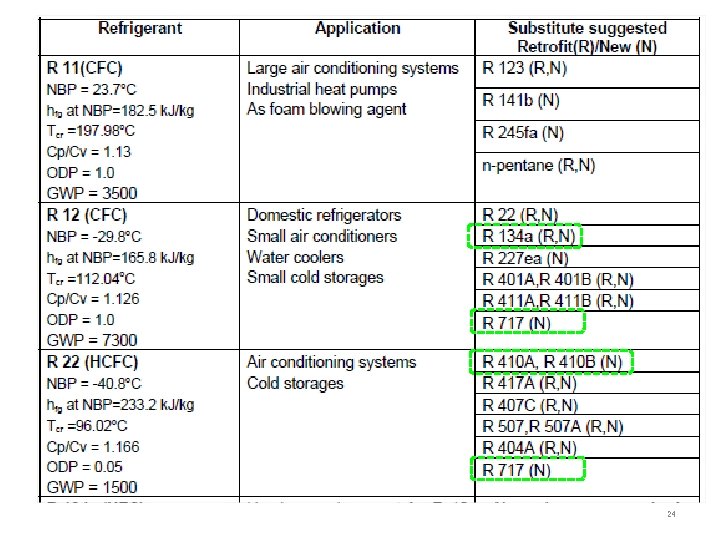
3. 2. 3 Environmental properties Mid-1970 s – alarm of ozone reduction in composition by CFC ODP Ozone Depletion Potential more UV radiation to earth Stop halocarbon as cause cancer aerosol spray propellant Montreal protocol ODP = 0 in foam insulation R 11 R 12 (Freon-12, CFC-12) first widely used, boiling point (23, -30) C – phased-out R 22 boiling point (-40) C - alternative to the highly ozonedepleting CFC-11 and CFC-12 will be phased-out in near-future In 1994, R 12 replaced by R 134 a, R-409 A blend R 22 replaced by HFCs, R 410, R 404 A, R 717, R 290(propane), R 409 A, R 438 A, R 507 A GWP: Global Warming Potential R 134 a: low ODP but high GWP be regulated in future TEWI: Total Equivalent Warming Index Direct & indirect effect to global warming 12

3. 2. 4 Economic properties The refrigerant used should preferably be - inexpensive and - easily available. R-22, retrofit using substitute refrigerants R-407 A is for use in for low- and medium-temp refrigeration. Uses a POE oil. R-421 A is for use in “air conditioning split systems, heat pumps, supermarket pak systems, dairy chillers, reach-in storage, bakery applications, refrigerated transport, self-contained display cabinets, and walk-in coolers. ” R-427 A is for use in air conditioning and refrigeration applications. It does not require all the mineral oil to be removed. It works with MO, AB, and POE oils. R-434 A is for use in water cooled and process chillers for air conditioning and medium- and low-temperature applications. It works with MO, AB, and POE oils. R-410 A: is for use in air conditioning and refrigeration applications R 32: new friendly-environment, low GWP, 2012 at Japan by Daikin 13

3. 3 Designation of refrigerants 3. 3. 4 Mixtures 3. 3. 1 Halocarbon compounds R 11, R 12, R 502 R 22 R 32, R 134 A, R 410 A 3. 3. 2 Inorganic compounds 14

3. 3. 1 Halocarbon compounds -- one or more of three halogens chlorine, fluorine and bromine x x x C H F -1 +1 First digit – number of fluorine atoms Second digit – one more than number of hydrogen atoms Third digit – one less than number of carbon atoms, 0 - omitted 15

3. 3. 2 Inorganic compounds Ammonia (NH 3) : R 717 Water (H 2 O) : R 718 Air : R 729 Carbon dioxide (CO 2) : R 44 Sulfer dioxide (SO 2) : R 764 organic compound- สปก. อนทรย เชอวามในสงมชวต สวนใหญม carbon ยกเวน COcompound. CO 2 carbonate inorganic สปก. อนนทรย เชอวาไมไดเกดขนในสงมช วต ไมมพนธะอะตอม carbon & hydrogen that contains A chlorofluorocarbon (CFC) is an organic compound only carbon, chlorine, and fluorine Because of ozone depletion, CFCs are being replaced with hydrofluorocarbons (HFCs) (e. g. , R-410 A, R 134 a), hydrocarbons (propane and iso-butane), and inorganic refrigerants (ammonia, CO 2) 16

3. 3. 3 Azeotropes and Zeotropic Mixtures a mixture of two or more liquids whose proportions cannot be altered by simple distillation also called -- constant boiling mixtures Greek words -- "no change on boiling" Most popular azeotrope: R 502 -- mixture of R 22 -48. 8% + R 115 -51. 2% Azeotropic mixtures: R 500: Mixture of R 12 (73. 8 %) and R 152 a (26. 2%) R 502: Mixture of R 22 (48. 8 %) and R 115 (51. 2%) R 503: Mixture of R 23 (40. 1 %) and R 13 (59. 9%) R 507 A: Mixture of R 125 (50%) and R 143 a (50%) Zeotropic mixtures: R 404 A : Mixture of R 125 (44%), R 143 a (52%) and R 134 a (4%) R 407 A : Mixture of R 32 (20%), R 125 (40%) and R 134 a (40%) R 407 B : Mixture of R 32 (10%), R 125 (70%) and R 134 a (20%) R 410 A : Mixture of R 32 (50%) and R 125 (50%) 17

รหสสถงสารทำความเยน โดย วศวกรรมสถานแหงประเทศไทย chlorofluorocarbons )วสท(. (CFCs) R-32 hydrochlorofluorocarbons (HCFCs) hydrofluorocarbons (HFCs) 18

3. 3. 4 Hydrocarbons Methane (CH 4) : R 50 Ethane (C 2 H 6) : R 170 Propane (C 3 H 8) : R 290 n-butane (C 4 H 10) : R 600 iso-butane (C 4 H 10) : R 600 a A hydrocarbon is an organic compound consisting entirely of hydrogen and carbon -low ODP but high GWP Unsaturated Hydrocarbons: R 1150 (C 2 H 4) R 1270 (C 3 H 6) 19

3. 5 Comparison between different refrigerants Start searching of Refrigerants volatile Refrigerants development (1) 1755 – water, water salt High operating pressure 1800 – ethyl ether (NBP +34. 5 C) Toxicity Flammability 1864 – dimethyl ether (NBP -23. 6 C) Toxicity 1866 – CO 2 compressor (low Tcr +31. 7 C) High operating pressure 1872 – NH 3 compressor (NBP -33. 3 C) Not compatible with copper 1874 – SO 2 compressor (NBP -10 C), auto-lubricant High corrosive 20

The synthetic CFCs/HCFCs 1928 Combination of 8 elements: carbon, nitrogen, oxygen, sulphur, hydrogen, fluorine, chlorine and bromine. Safe refrigerant Low operating pressure Non-toxicity Inflammability 1800 -1866 60 years of ethyl ether 1931 – Freon-12 Replace CH 4 by CCl 2 F 2 Freon-11 Freon-22 inorganic – R 7 xx 1866 -1930 60 years of CO 2 NH 3 SO 2 1931 -1987 56 years of CFCs Refrigerant mixture – R 4 xx (zeotropic), R 5 xx (azeotropic) 1988 - 2020: 30 years of refrigerant development i) Non-ODS, synthetic refrigerants based on Hydro-Fluoro-Carbons (HFCs) and their blends ii) Natural refrigerants including ammonia, carbon dioxide, hydrocarbons and their blends 21

Basic of choice of refrigerants Air (R 718) Used in aircraft, safe, light weight compensate for low COP Ammonia Mostly used, Large industrial, low-temp. , low price, Absorption (R 717) Used for direct-contact freezing of food CO 2 (R 744) High p Limit to low-temp. side of cascade system c R 11 Along with R 113, popular for centrifugal compressor systems R 12 reciprocating compressor – domestic refrigeration, automotive-air-cond. R 22 Smaller and lower-cost compressor than R 12 – air conditioning R 502 Advantages of R 22 + better behavior with oil + lower tdischarge R 114 Rotary compressor, Absorption 22

23

24

25

26

ขอสรปของนำยา R 32 เมอเทยบกบ R 32 R 410 A และR 22 R 410 A 1. ODP & GWP R 11, R 12 R 22 R 410 A R 32 2. Cost R 410 A 700 -800 บาท/kg R 22 & R 32 300 -400 บาท/kg R 22 27
