Topic 3 Periodicity 3 1 Periodic Table 1

Topic 3 - Periodicity 3. 1 – Periodic Table . 1
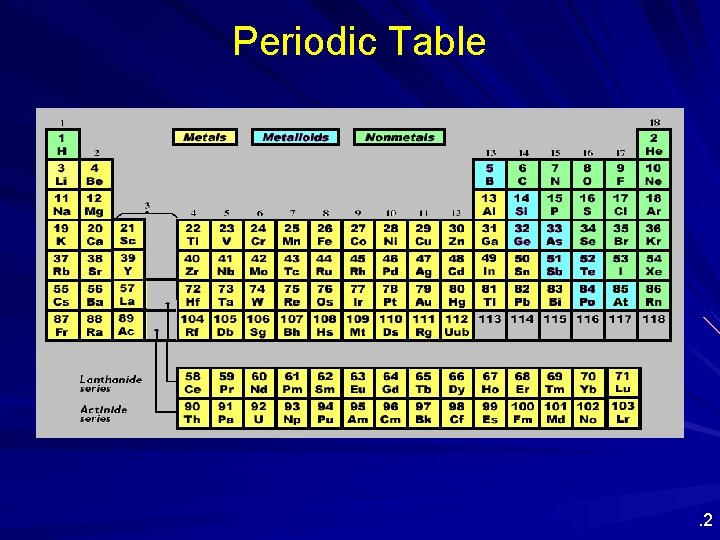
Periodic Table . 2
The Periodic Table-Key Questions What is the periodic table ? What information does the table provide ? How can one use the periodic table to predict the properties of the elements? . 3
Periodic Table • The development of the periodic table brought a system of order to what was otherwise an collection of thousands of pieces of information. • The periodic table is a milestone in the development of modern chemistry. It not only brought order to the elements but it also enabled scientists. to predict the existence of elements that had not yet been discovered. . 4
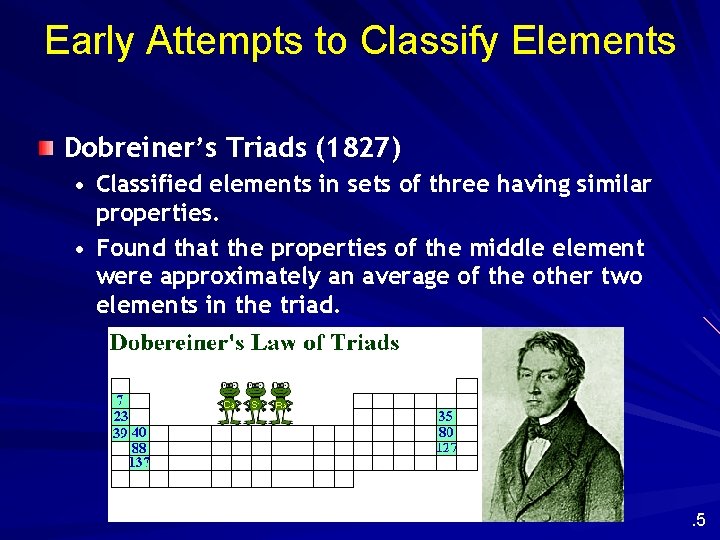
Early Attempts to Classify Elements Dobreiner’s Triads (1827) • Classified elements in sets of three having similar properties. • Found that the properties of the middle element were approximately an average of the other two elements in the triad. . 5
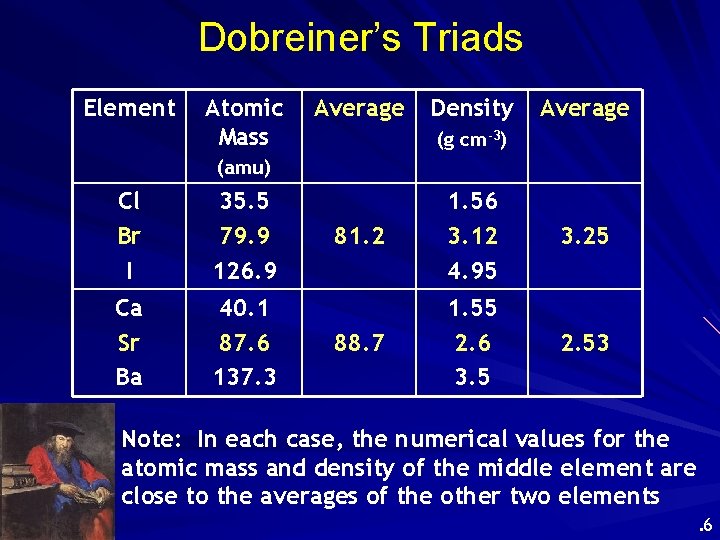
Dobreiner’s Triads Element Atomic Mass Average Density Average (g cm-3) (amu) Cl Br I 35. 5 79. 9 126. 9 Ca Sr Ba 40. 1 87. 6 137. 3 81. 2 1. 56 3. 12 4. 95 3. 25 88. 7 1. 55 2. 6 3. 5 2. 53 Note: In each case, the numerical values for the atomic mass and density of the middle element are close to the averages of the other two elements. 6

Newland’s Octaves -1863 John Newland attempted to classify then 62 known elements of his day. He observed that when classified according to atomic mass, similar properties appeared to repeat for about every eighth element His Attempt to correlate the properties of elements with musical scales subjected him to ridicule. In the end his work was acknowledged and he was vindicated with the award of the Davy Medal in 1887 for his work. . 7

Dmitri Mendeleev is credited with creating the modern periodic table of the elements. He gets the credit because he not only arranged the atoms, but he also made predictions based on his arrangements His predictions were later shown to be quite accurate. . 8
Mendeleev’s Periodic Table • Mendeleev organized all of the elements into one comprehensive table. • Elements were arranged in order of increasing mass. • Elements with similar properties were placed in the same row. . 9
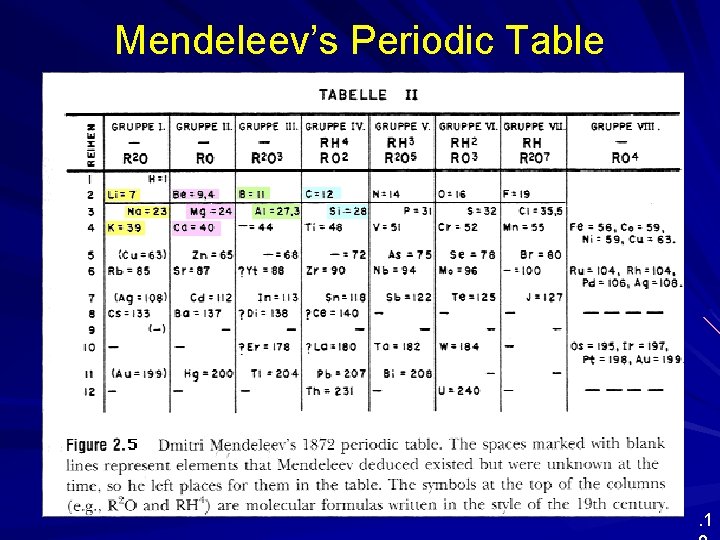
Mendeleev’s Periodic Table . 1
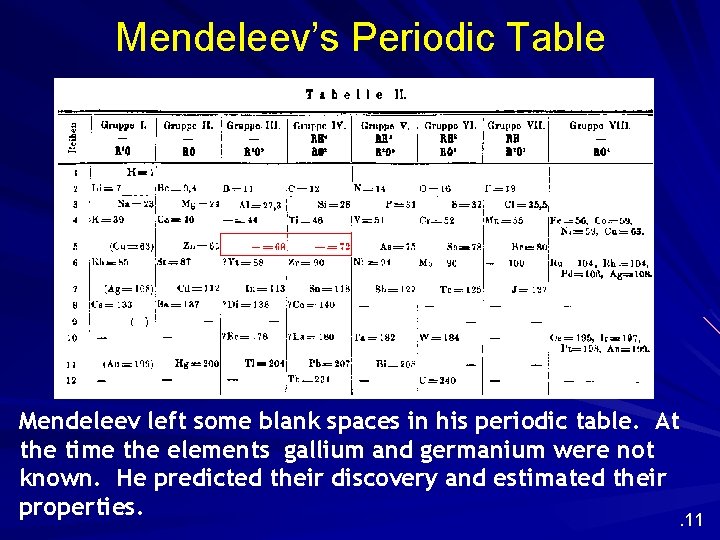
Mendeleev’s Periodic Table Mendeleev left some blank spaces in his periodic table. At the time the elements gallium and germanium were not known. He predicted their discovery and estimated their properties. . 11
The Modern Periodic Table The Periodic Table has undergone several modifications before it evolved in its present form. The current form is usually attributed to Glenn Seaborg in 1945. . 12
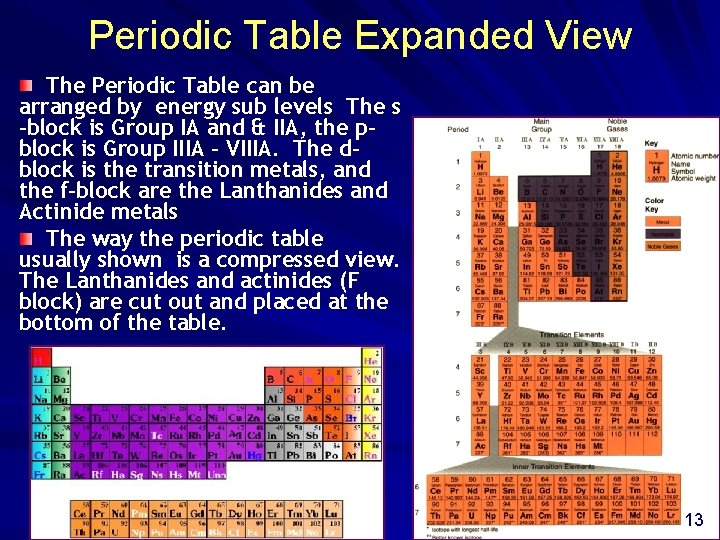
Periodic Table Expanded View The Periodic Table can be arranged by energy sub levels The s -block is Group IA and & IIA, the pblock is Group IIIA - VIIIA. The dblock is the transition metals, and the f-block are the Lanthanides and Actinide metals The way the periodic table usually shown is a compressed view. The Lanthanides and actinides (F block) are cut out and placed at the bottom of the table. . 13
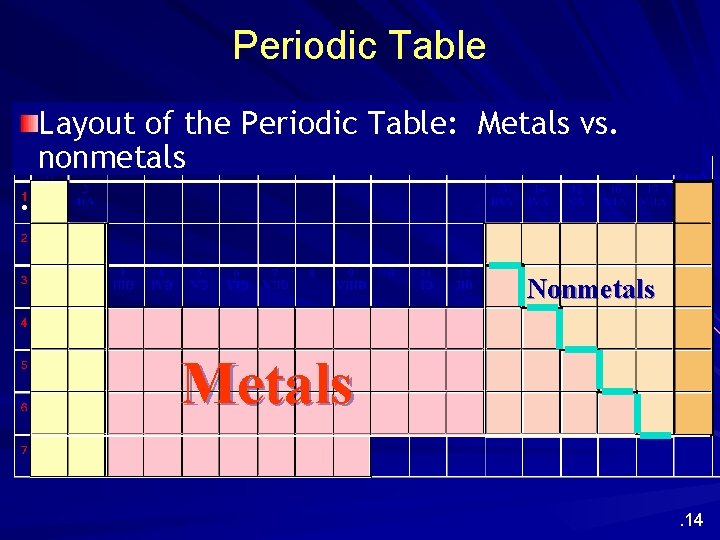
Periodic Table Layout of the Periodic Table: Metals vs. nonmetals. Nonmetals Metals. 14
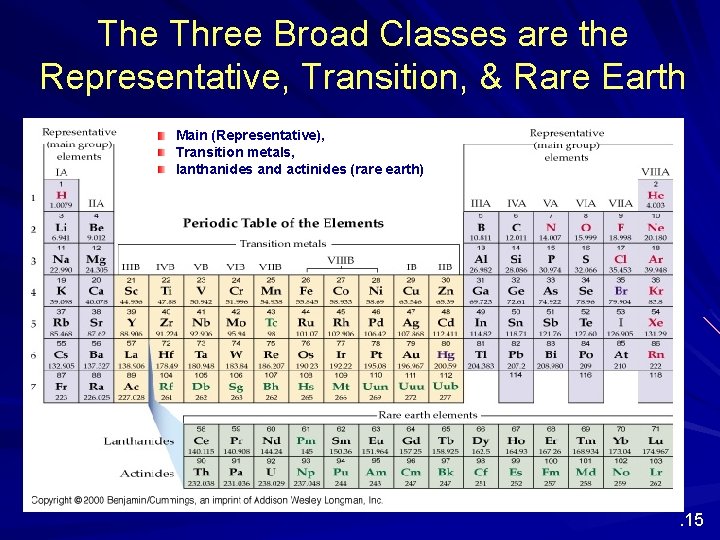
The Three Broad Classes are the Representative, Transition, & Rare Earth Main (Representative), Transition metals, lanthanides and actinides (rare earth) . 15
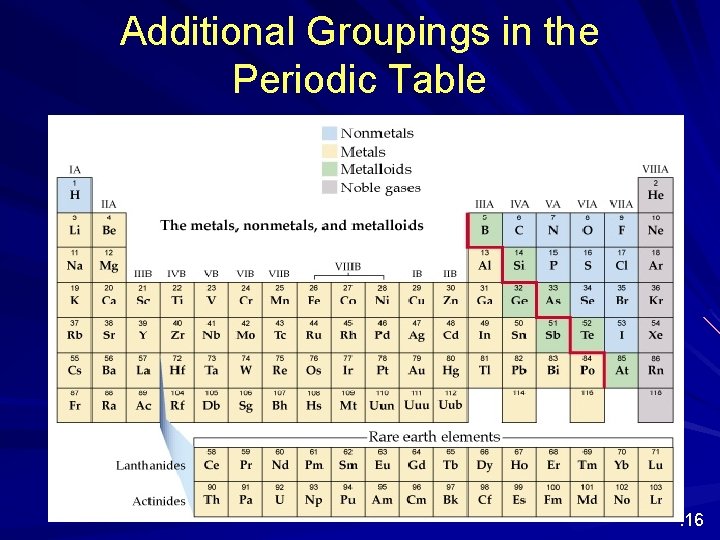
Additional Groupings in the Periodic Table Nonmetals, Metalloids, Noble gases . 16
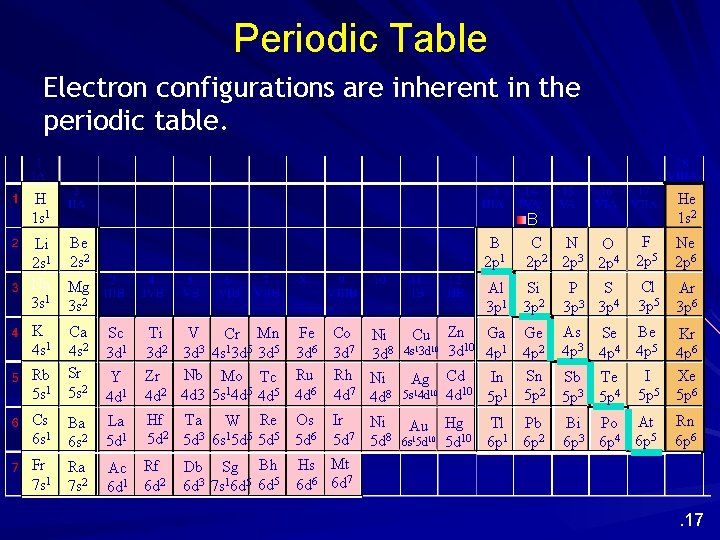
Periodic Table Electron configurations are inherent in the periodic table. H 1 s 1 Li Be 2 s 1 2 s 2 Na Mg 3 s 1 3 s 2 K 4 s 1 Sc 3 d 1 Rb 5 s 1 Ca 4 s 2 Sr 5 s 2 Y 4 d 1 V Ti Cr Mn Fe Co 3 d 2 3 d 3 4 s 13 d 5 3 d 6 3 d 7 Zr Nb Mo Tc Ru Rh 4 d 2 4 d 3 5 s 14 d 5 4 d 6 4 d 7 Cs 6 s 1 Ba 6 s 2 La 5 d 1 Hf Ta W Re Os 5 d 2 5 d 3 6 s 15 d 5 5 d 6 Fr 7 s 1 Ra 7 s 2 Ac Rf 6 d 1 6 d 2 Db Sg Bh 6 d 3 7 s 16 d 5 Ni 3 d 8 Ni 4 d 8 Ir Ni 7 5 d 5 d 8 Hs Mt 6 d 6 6 d 7 Cu 4 s 13 d 10 Ag 5 s 14 d 10 Au 6 s 15 d 10 He 1 s 2 B 2 p 1 B 1 2 p C N O 2 3 2 p 2 p 2 p 4 F 2 p 5 Ne 2 p 6 Al 3 p 1 Si 3 p 2 S P 3 3 p 3 p 4 Cl 3 p 5 Ar 3 p 6 Zn Ga Ge 3 d 10 4 p 1 4 p 2 Cd In Sn 10 4 d 5 p 1 5 p 2 As Se 4 p 3 4 p 4 Be 4 p 5 Sb Te 5 p 3 5 p 4 I 5 p 5 Kr 4 p 6 Xe 5 p 6 Hg Tl Pb 5 d 10 6 p 1 6 p 2 Bi Po At 6 p 3 6 p 4 6 p 5 Rn 6 p 6 . 17
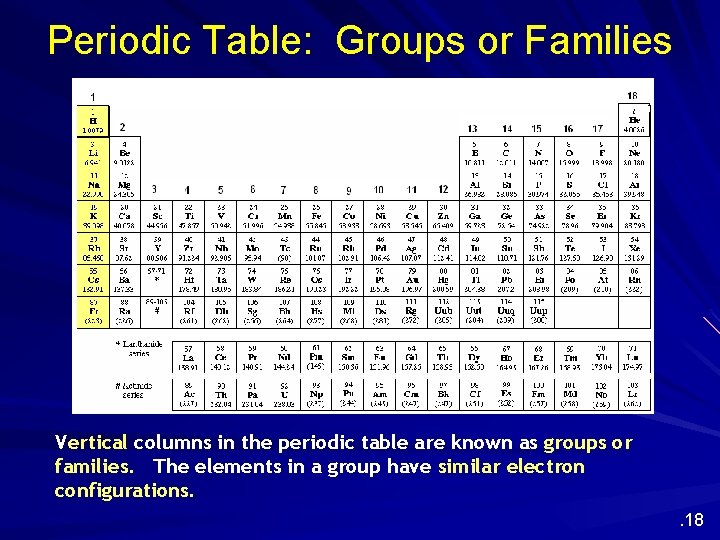
Periodic Table: Groups or Families Vertical columns in the periodic table are known as groups or families. The elements in a group have similar electron configurations. . 18
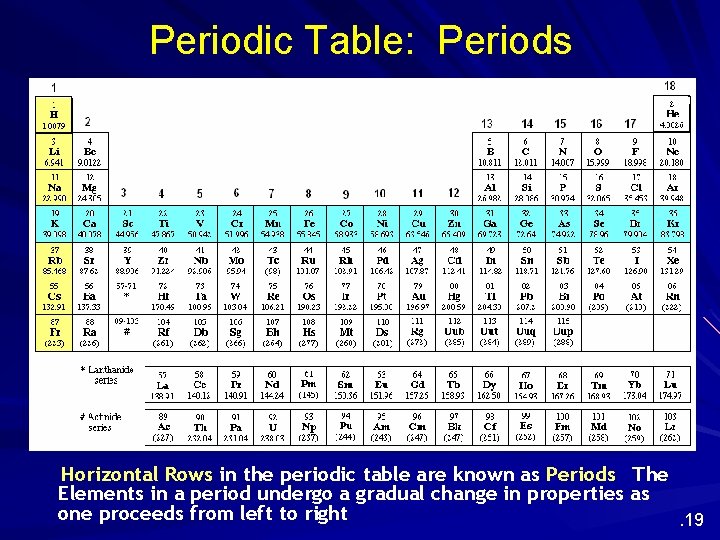
Periodic Table: Periods Horizontal Rows in the periodic table are known as Periods The Elements in a period undergo a gradual change in properties as one proceeds from left to right. 19

Alternate Periodic Tables Although we are most familiar with the periodic table that Seaborg proposed more than 60 years ago, several alternate designs have been proposed. . 20
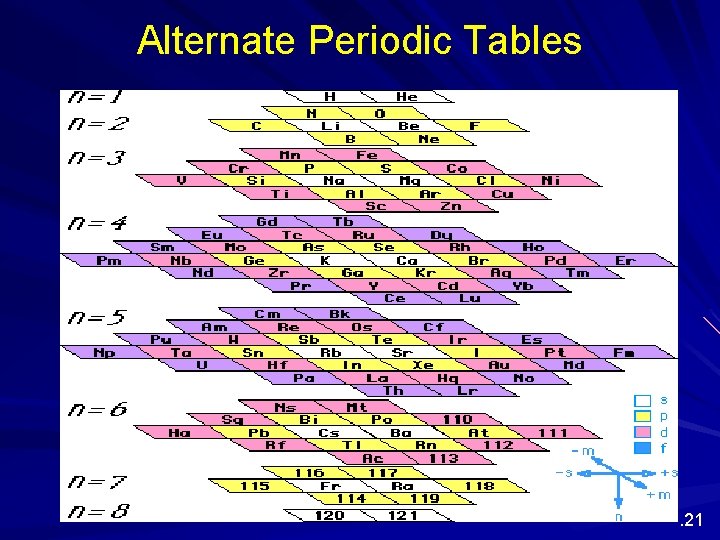
Alternate Periodic Tables . 21
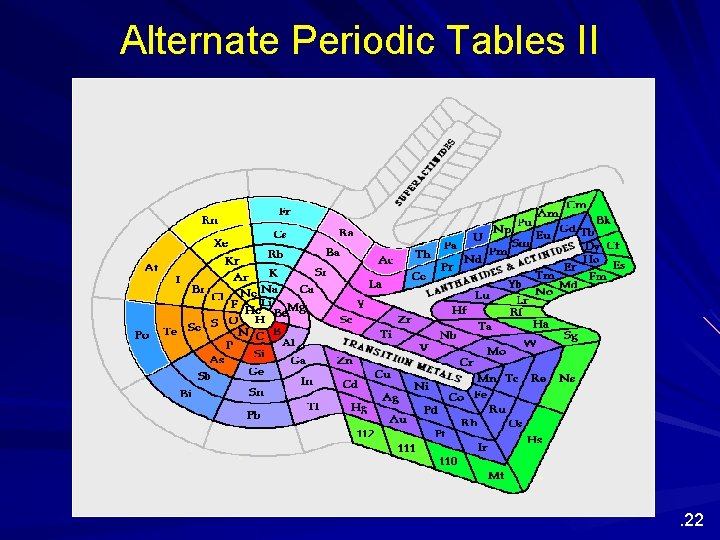
Alternate Periodic Tables II . 22
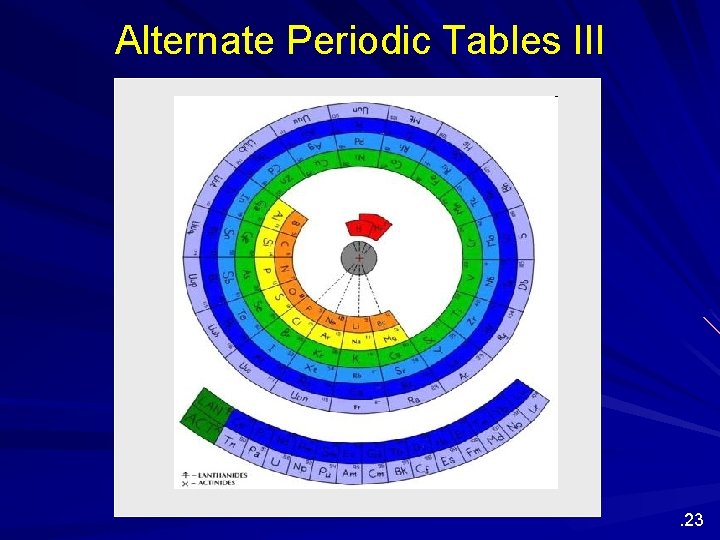
Alternate Periodic Tables III . 23
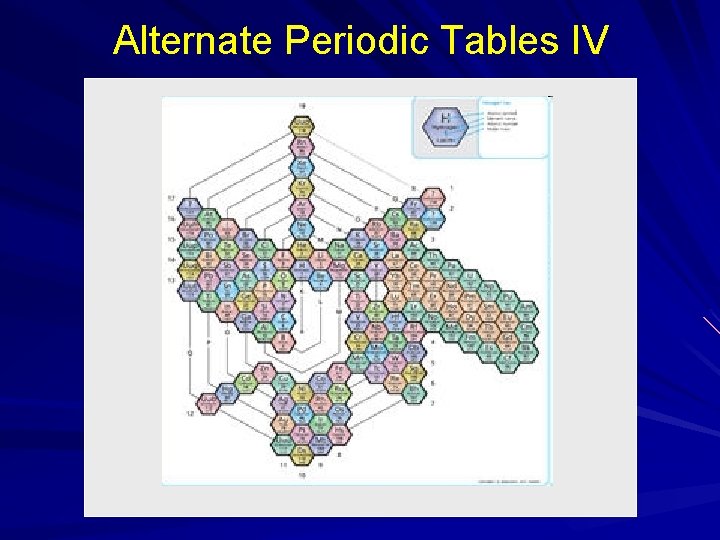
Alternate Periodic Tables IV
- Slides: 24