Topic 3 Cell Membrane OBJ 9 Phospholipids Primary

Topic 3 Cell Membrane
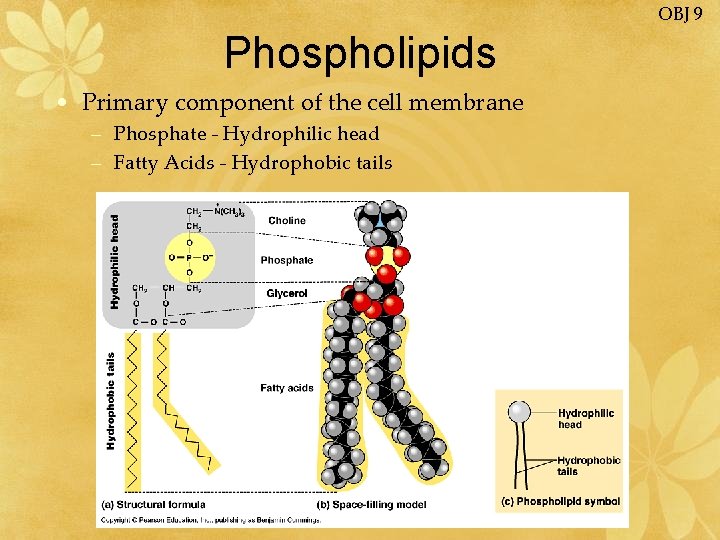
OBJ 9 Phospholipids • Primary component of the cell membrane – Phosphate - Hydrophilic head – Fatty Acids - Hydrophobic tails
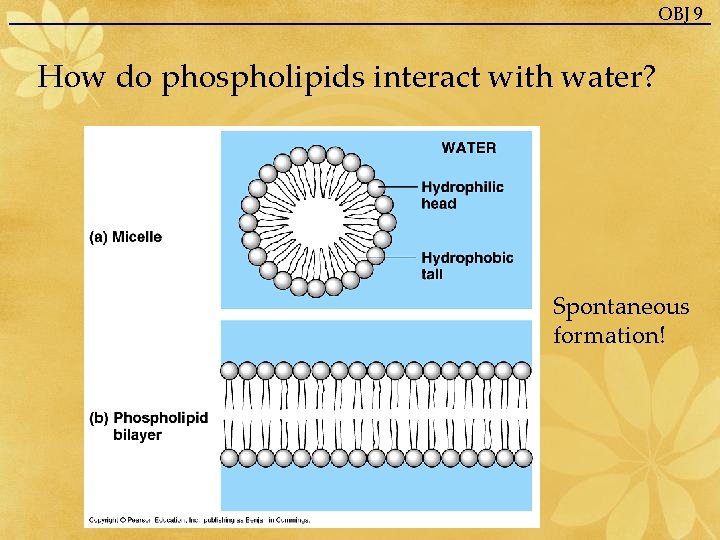
OBJ 9 How do phospholipids interact with water? Spontaneous formation!
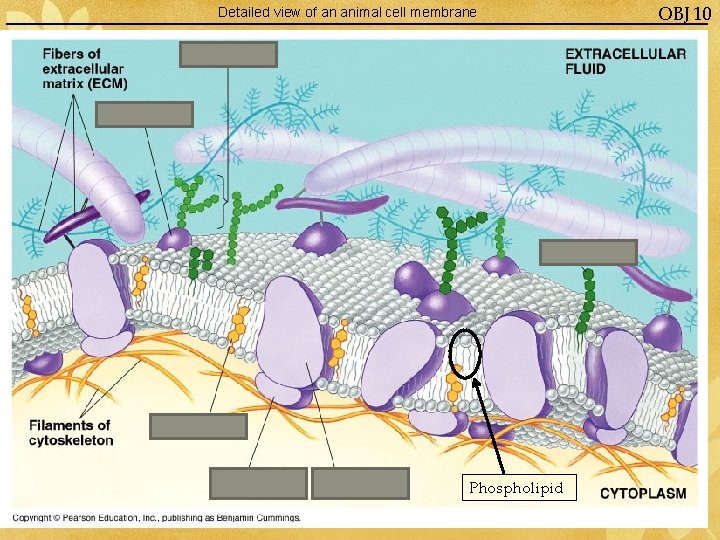
Detailed view of an animal cell membrane Phospholipid OBJ 10
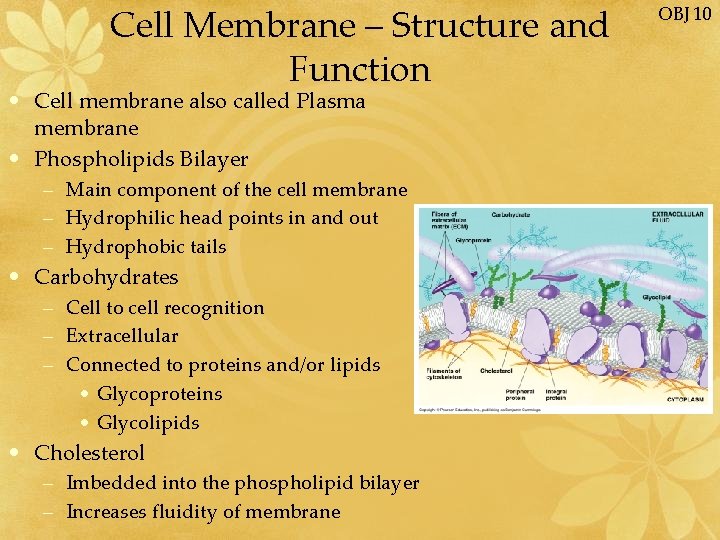
Cell Membrane – Structure and Function • Cell membrane also called Plasma membrane • Phospholipids Bilayer – Main component of the cell membrane – Hydrophilic head points in and out – Hydrophobic tails • Carbohydrates – Cell to cell recognition – Extracellular – Connected to proteins and/or lipids • Glycoproteins • Glycolipids • Cholesterol – Imbedded into the phospholipid bilayer – Increases fluidity of membrane OBJ 10
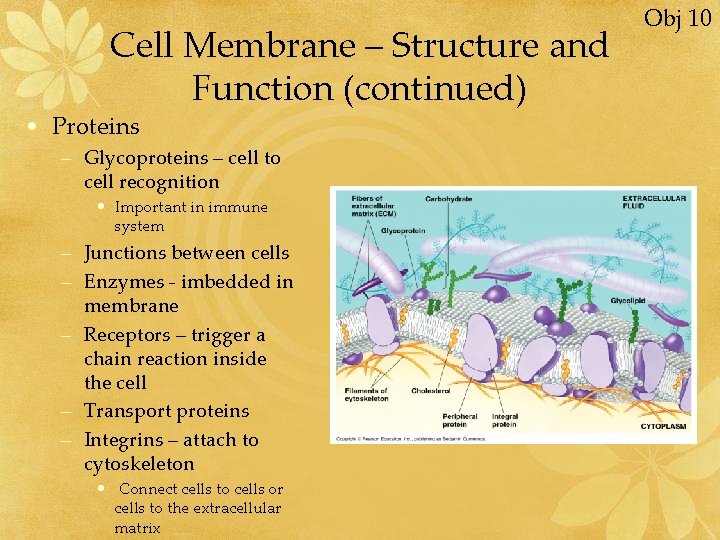
Cell Membrane – Structure and Function (continued) • Proteins – Glycoproteins – cell to cell recognition • Important in immune system – Junctions between cells – Enzymes - imbedded in membrane – Receptors – trigger a chain reaction inside the cell – Transport proteins – Integrins – attach to cytoskeleton • Connect cells to cells or cells to the extracellular matrix Obj 10
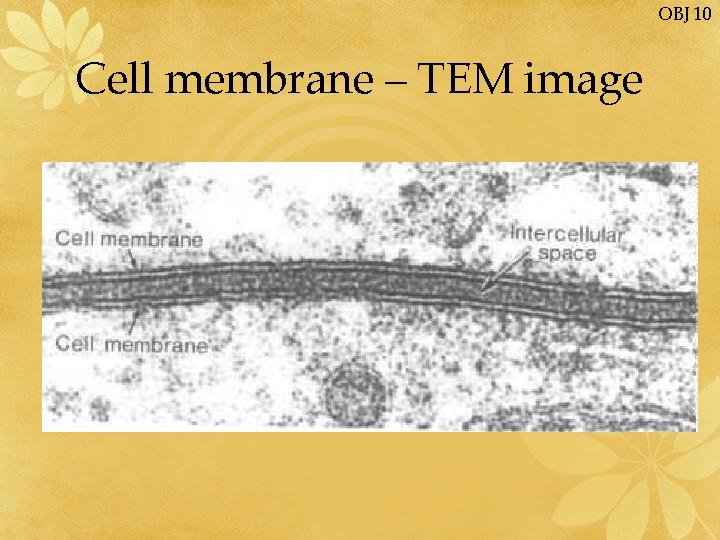
OBJ 10 Cell membrane – TEM image
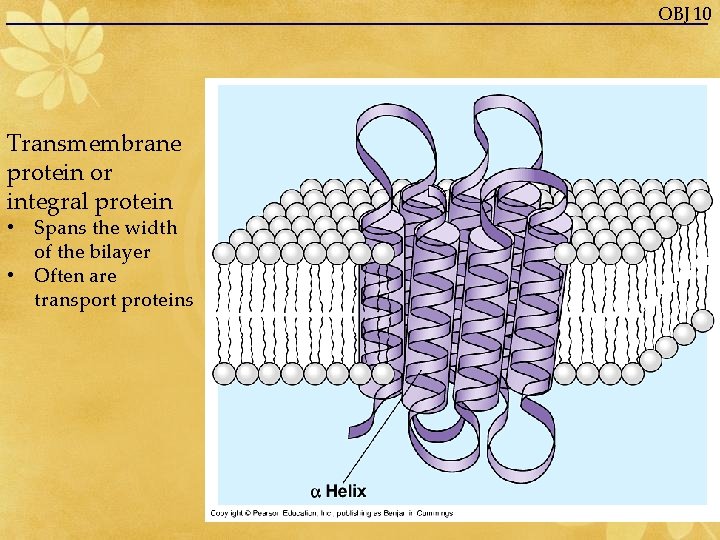
OBJ 10 Transmembrane protein or integral protein • Spans the width of the bilayer • Often are transport proteins
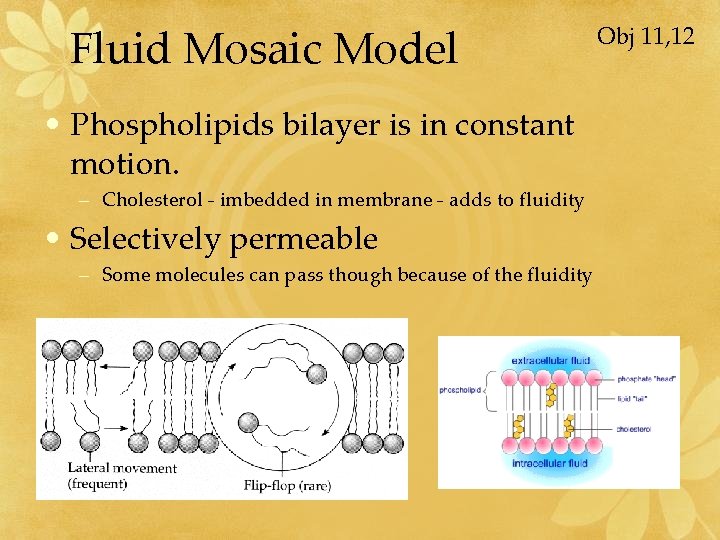
Fluid Mosaic Model • Phospholipids bilayer is in constant motion. – Cholesterol - imbedded in membrane - adds to fluidity • Selectively permeable – Some molecules can pass though because of the fluidity Obj 11, 12

Cell membranes – “the gate keeper of the cell” • What does a cell need to bring in? • What does a cell need to get rid of?
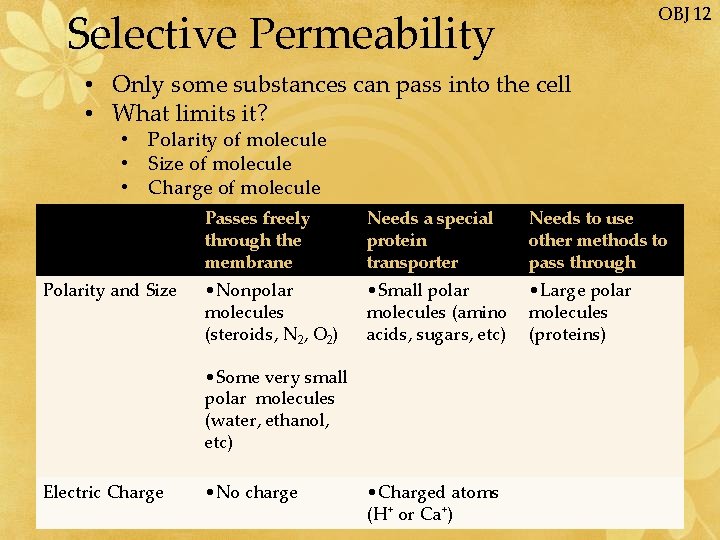
OBJ 12 Selective Permeability • Only some substances can pass into the cell • What limits it? • • Polarity of molecule Size of molecule Charge of molecule Size Passes freely Polarity and Size through the membrane Needs a special protein transporter Needs to use other methods to pass through • Nonpolar molecules (steroids, N 2, O 2) • Small polar molecules (amino acids, sugars, etc) • Large polar molecules (proteins) • Some very small polar molecules (water, ethanol, etc) Electric Charge • No charge • Charged atoms (H+ or Ca+)
- Slides: 11