Topic 2 Speed of Reactions Topic 2 Speed














- Slides: 16

Topic 2 - Speed of Reactions

Topic 2 - Speed of Reactions Chemical reactions can be fast: Like this explosion

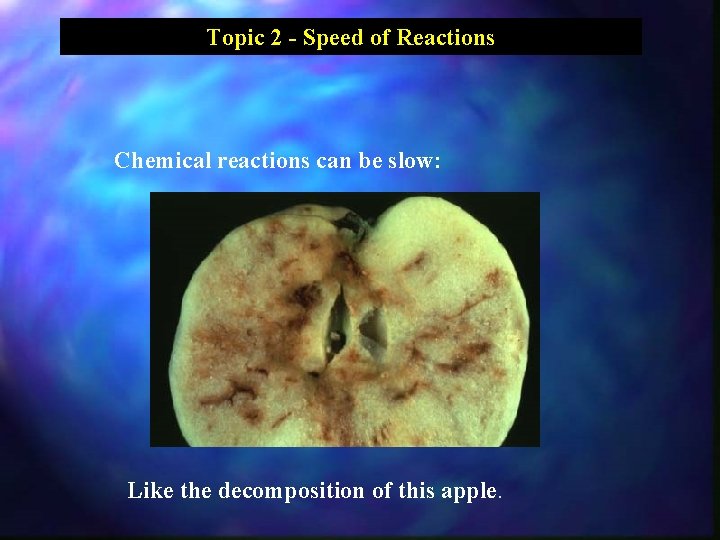
Topic 2 - Speed of Reactions Chemical reactions can be slow: Like the decomposition of this apple.

Topic 2 - Speed of Reactions Chemical reactions can be very slow: Like the rusting of iron.

Topic 2 - Speed of Reactions We can speed up chemical reactions: By increasing the temperature. When heated the particles of substances: have more energy move faster collide more often are more likely to react successfully.

Topic 2 - Speed of Reactions We can speed up chemical reactions: By increasing the concentration. • A more concentrated solution has more reacting particles • Particles have a bigger chance of coming into contact and reacting.

Topic 2 - Speed of Reactions We can speed up chemical reactions: By decreasing the particle size. When a lump of a reacting substance is broken into smaller pieces: • the substance can more easily come into contact with the other reactant • material from the inside of the lump can now react not just the outside surface.

Topic 2 - Speed of Reactions There are many examples of reactions which are affected by temperature. • Glucose reacts more quickly with Benedict’s solution as the temperature is increased. • Rusting of ships sunk in deep cold waters takes place very slowly. • Decay of food speeds up when it is removed from the fridge into a higher temperature. • Stains are more easily removed from washing at higher temperatures.

Topic 2 - Speed of Reactions There are many examples of reactions which are affected by changing concentration. • Clothes can be bleached more quickly using a more concentrated bleach. • Magnesium dissolves quicker and gives off hydrogen faster in 2% acid than it does in 1% acid. • When the amount of washing powder is less, clothes are not cleaned so effectively because the reaction is slower.

Topic 2 - Speed of Reactions There are many examples of reactions which are affected by changing particle size. • Chalk lumps react with acid much more slowly than chalk powder.

Topic 2 - Speed of Reactions Smaller particle size speeds up reaction. • Potatoes cut into smaller pieces cook more quickly than whole potatoes.

Topic 2 - Speed of Reactions Large surface area - quick reaction • Iron powder blown into a flame burns rapidly while an iron bar just glows.

Topic 2 - Speed of Reactions Catalysts • Speed up chemical reactions • Are not used up in the reaction • Can be recovered at the end of the reaction. • Save energy because they allow many reactions to be carried out at a lower temperature.

Topic 2 - Speed of Reactions Catalysts have many uses: • Iron is used as a catalyst in the production of ammonia, an important chemical used in fertiliser production. • Nickel is used in hydrogenation of vegetable oils. This process hardens oils to make margarine. Note: the catalysts above are transition metals.

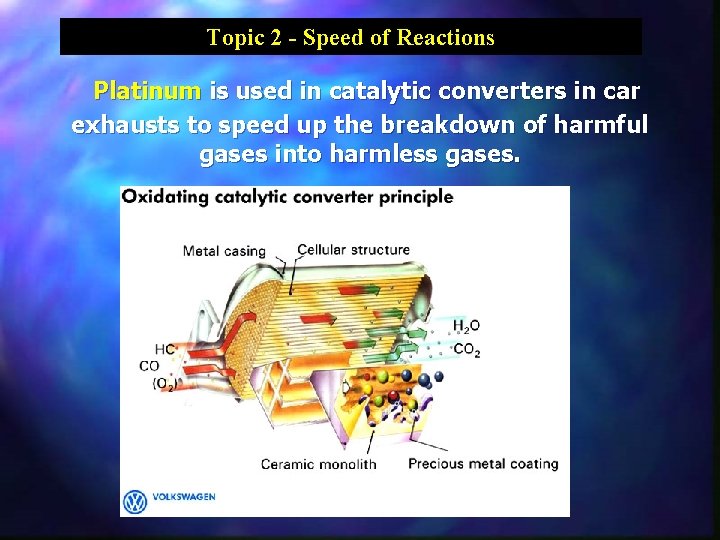
Topic 2 - Speed of Reactions Platinum is used in catalytic converters in car exhausts to speed up the breakdown of harmful gases into harmless gases.

Topic 2 - Speed of Reactions Enzymes • are biological catalysts • speed up reactions in living things. • In the human body reactions have to take place at 370 C and many are helped by enzymes. • Amylase is an enzyme in our saliva which speeds up the breakdown of starch. • Enzymes are used in the production of beer, wine, cheese and yoghurt.