Topic 2 2 WATER Topic Outline AUDIO Structure


- Slides: 13

Topic 2. 2 WATER

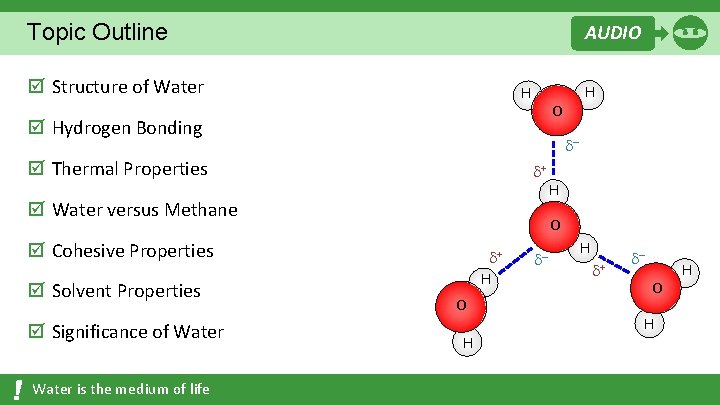
Topic Outline AUDIO þ Structure of Water H O þ Hydrogen Bonding – þ Thermal Properties + þ Water versus Methane þ Significance of Water is the medium of life H O þ Cohesive Properties þ Solvent Properties H + H O H – H + – O H H

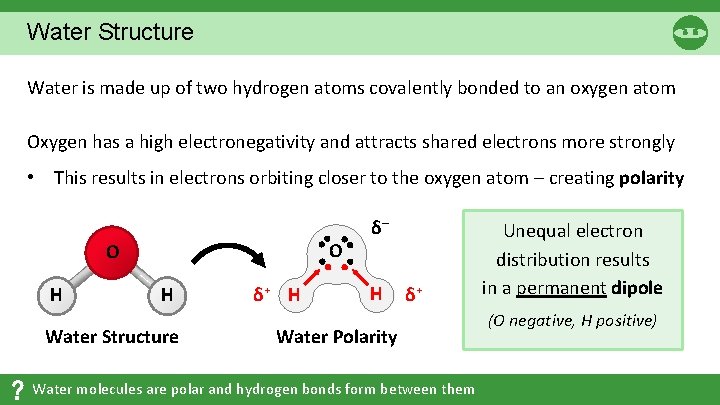
Water Structure Water is made up of two hydrogen atoms covalently bonded to an oxygen atom Oxygen has a high electronegativity and attracts shared electrons more strongly • This results in electrons orbiting closer to the oxygen atom – creating polarity O O H H Water Structure δ+ H δ– H δ+ Water Polarity Water molecules are polar and hydrogen bonds form between them Unequal electron distribution results in a permanent dipole (O negative, H positive)

Hydrogen Bonding The dipolarity of a water molecule allows it to form polar associations with other charged molecules (polar or ionic) Hydrogen bonds are particularly strong polar associations that δ– δ+ form between hydrogen and either fluorine, oxygen or nitrogen • This is because H atoms have a very weak electronegativity, while F, O & N atoms have a very strong electronegativity Water molecules will collectively form hydrogen bonds (H + O) Water molecules are polar and hydrogen bonds form between them H bonds δ+ δ–

Properties of Water Intermolecular bonding between water molecules give water distinct properties: Thermal Properties • Water can absorb significant amounts of heat energy before changing state Cohesive / Adhesive Properties • Water ‘sticks’ to water molecules (cohesion) and charged substances (adhesion) Solvent Properties • Water dissolves polar / ionic substances (making it an effective transport medium) Hydrogen bonds and dipolarity explains the cohesive, adhesive, thermal and solvent properties of water

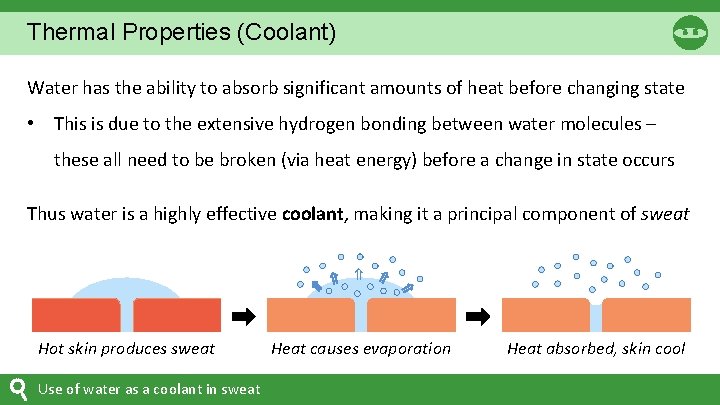
Thermal Properties (Coolant) Water has the ability to absorb significant amounts of heat before changing state • This is due to the extensive hydrogen bonding between water molecules – these all need to be broken (via heat energy) before a change in state occurs Hot skin produces sweat Use of water as a coolant in sweat ⇒ ⇒ Thus water is a highly effective coolant, making it a principal component of sweat ⇒ Heat causes evaporation Heat absorbed, skin cool

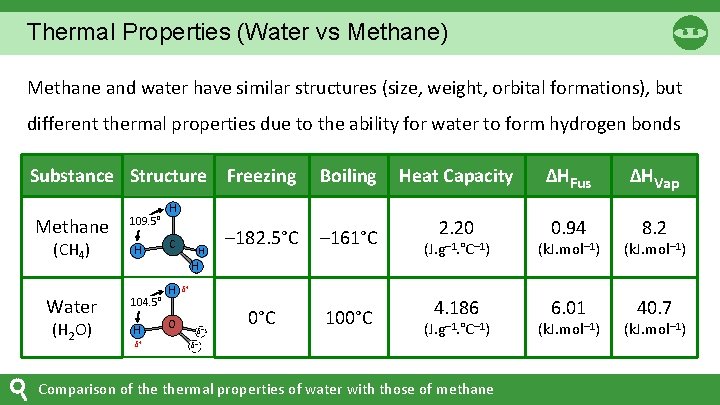
Thermal Properties (Water vs Methane) Methane and water have similar structures (size, weight, orbital formations), but different thermal properties due to the ability for water to form hydrogen bonds Substance Structure Freezing Methane (CH 4) Water (H 2 O) 109. 5° H Boiling Heat Capacity ΔHFus ΔHVap – 182. 5°C – 161°C 2. 20 0. 94 8. 2 0°C 100°C H H 104. 5° H δ+ H O (J. g– 1. °C– 1) (k. J. mol– 1) 4. 186 6. 01 40. 7 δ+ δ– (J. g– 1. °C– 1) δ– Comparison of thermal properties of water with those of methane (k. J. mol– 1)

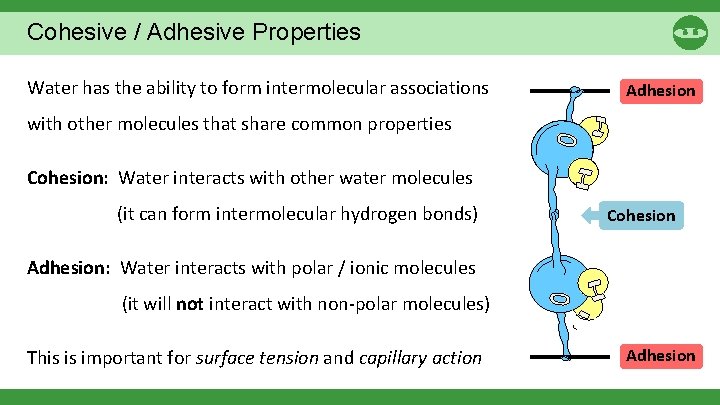
Cohesive / Adhesive Properties Water has the ability to form intermolecular associations Adhesion with other molecules that share common properties Cohesion: Water interacts with other water molecules (it can form intermolecular hydrogen bonds) Cohesion Adhesion: Water interacts with polar / ionic molecules (it will not interact with non-polar molecules) This is important for surface tension and capillary action Adhesion

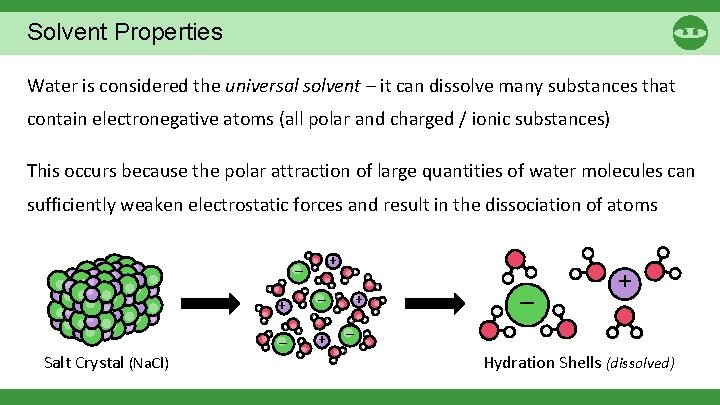
Solvent Properties Water is considered the universal solvent – it can dissolve many substances that contain electronegative atoms (all polar and charged / ionic substances) This occurs because the polar attraction of large quantities of water molecules can sufficiently weaken electrostatic forces and result in the dissociation of atoms + – Salt Crystal (Na. Cl) + – – + + – Hydration Shells (dissolved)

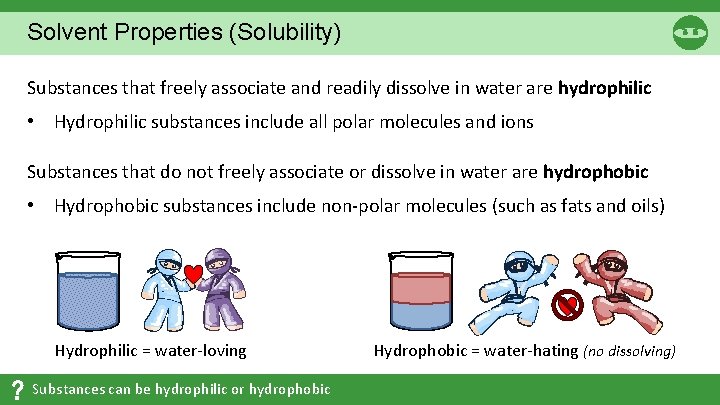
Solvent Properties (Solubility) Substances that freely associate and readily dissolve in water are hydrophilic • Hydrophilic substances include all polar molecules and ions Substances that do not freely associate or dissolve in water are hydrophobic • Hydrophobic substances include non-polar molecules (such as fats and oils) Hydrophilic = water-loving Substances can be hydrophilic or hydrophobic Hydrophobic = water-hating (no dissolving)

Solvent Properties (Transport) The transport of materials within the blood will depend upon their solubility Water Soluble Substances: • Sodium chloride (ionic) and glucose (polar) are freely transported in the blood • Amino acids are transported in an ionized state (amine / carboxyl group charged) • Oxygen is soluble in low amounts – generally transported in red blood cells Water Insoluble Substances: • Lipids (cholesterol, fat) are packaged with proteins (lipoproteins) for transport Modes of transport of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in blood

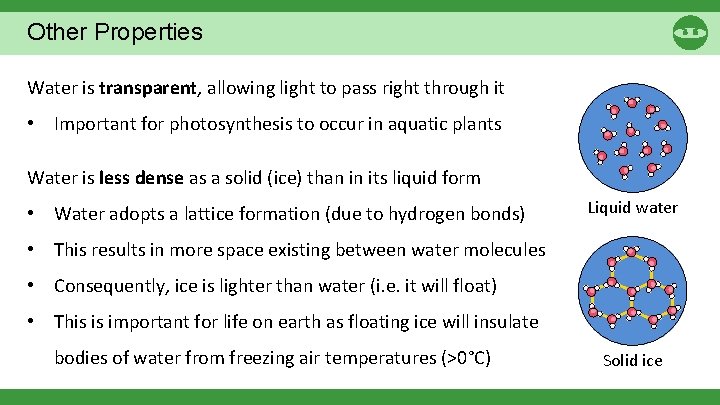
Other Properties Water is transparent, allowing light to pass right through it • Important for photosynthesis to occur in aquatic plants Water is less dense as a solid (ice) than in its liquid form • Water adopts a lattice formation (due to hydrogen bonds) Liquid water • This results in more space existing between water molecules • Consequently, ice is lighter than water (i. e. it will float) • This is important for life on earth as floating ice will insulate bodies of water from freezing air temperatures (>0°C) Solid ice

Topic Review Can you do the following? • Describe the molecular structure of water • Outline thermal properties of water • Compare the properties of water and methane • Distinguish between cohesion and adhesion • Identify hydrophilic and hydrophobic substances • Outline the solvent properties of water • Explain the significance of water to living things