Topic 2 1 Molecules to metabolism Essential idea
Topic 2. 1 Molecules to metabolism Essential idea: Living organisms control their composition by a complex web of chemical reactions.
2. 1 Molecules to metabolism Nature of science: Falsification of theories—the artificial synthesis of urea helped to falsify vitalism. (1. 9) Understandings • Molecular biology explains living processes in terms of the chemical substance involved • Carbon atoms can form four covalent bonds allowing a diversity of stable compounds to exist • Life is based on carbon compounds including carbohydrates, lipids, proteins, and nucleic acids • Metabolism is the web of all the enzyme-catalyzed reactions in a cell or organism • Anabolism is the synthesis of complex molecules from simpler molecules including the formation of macromolecules from monomers by condensation reactions • Catabolism is the breakdown of complex molecules into simpler molecules including the hydrolysis of macromolecules into monomers Applications and skills • Application: Urea as an example of a compound that is produced by living organisms but can also be artificially synthesized • Skill: Drawing molecular diagrams of glucose, ribose, a saturated fatty acid and a generalized amino acid • Skill: Identification of biochemical such as sugars, lipids, or amino acids from molecular diagrams
U 2. 1. 1 Molecular biology explains living processes in terms of the chemical substance involved • It was once believed that living and non-living things were made of different substances • What was theory of vitalism? • Theory that living organisms were made of organic chemicals that could only be found in living things because a vital force was needed • This was proven false • We now know that living and non-living organisms are composed of the same chemical and physical forces • What is a better explanation as to why living and non-living things are different? • Natural selection
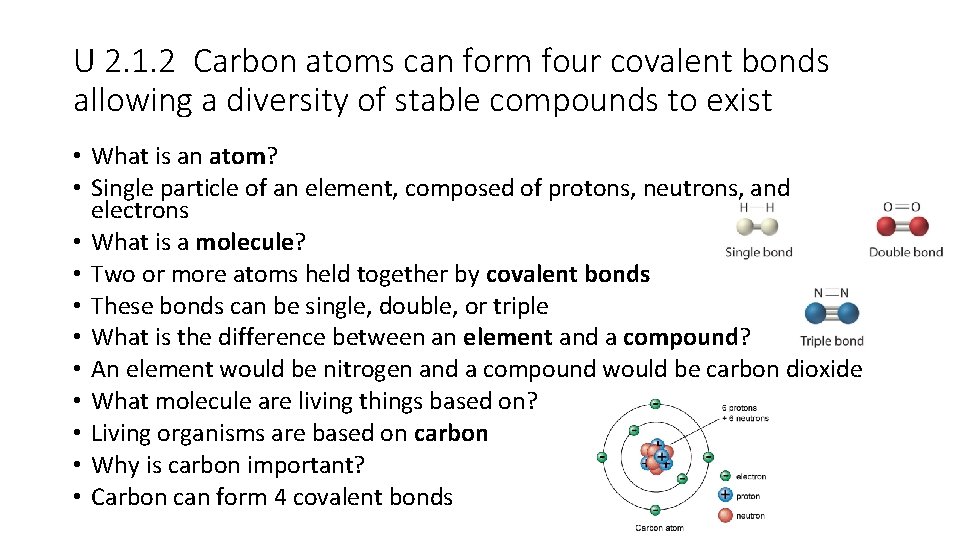
U 2. 1. 2 Carbon atoms can form four covalent bonds allowing a diversity of stable compounds to exist • What is an atom? • Single particle of an element, composed of protons, neutrons, and electrons • What is a molecule? • Two or more atoms held together by covalent bonds • These bonds can be single, double, or triple • What is the difference between an element and a compound? • An element would be nitrogen and a compound would be carbon dioxide • What molecule are living things based on? • Living organisms are based on carbon • Why is carbon important? • Carbon can form 4 covalent bonds
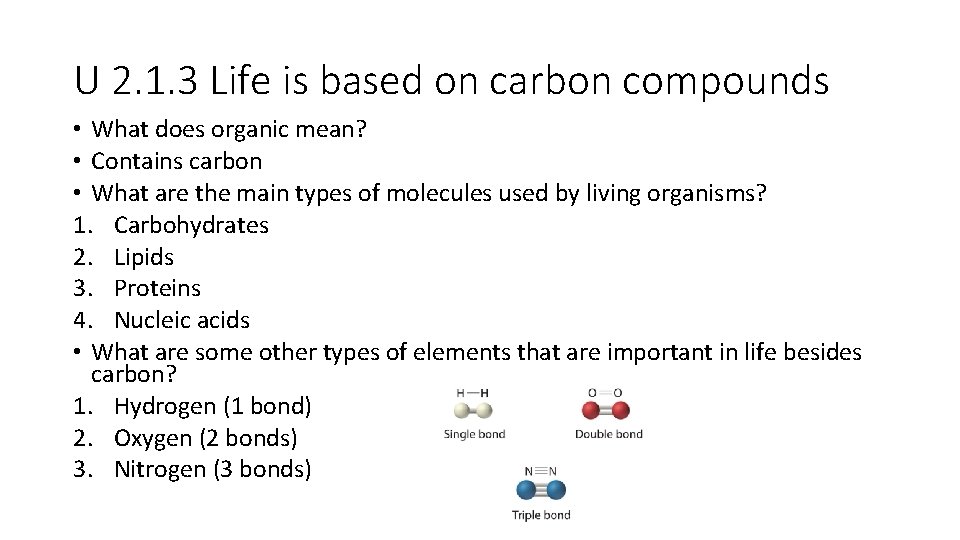
U 2. 1. 3 Life is based on carbon compounds • What does organic mean? • Contains carbon • What are the main types of molecules used by living organisms? 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic acids • What are some other types of elements that are important in life besides carbon? 1. Hydrogen (1 bond) 2. Oxygen (2 bonds) 3. Nitrogen (3 bonds)
U 2. 1. 4 Metabolism is the web of all the enzyme-catalyzed reactions in a cell or organism • What is metabolism? • Chemical reactions that maintain a living organism • What are metabolic pathways? • Series of chemical reactions catalyzes by enzymes where the product of one acts as a substrate for another • Chains or cycles
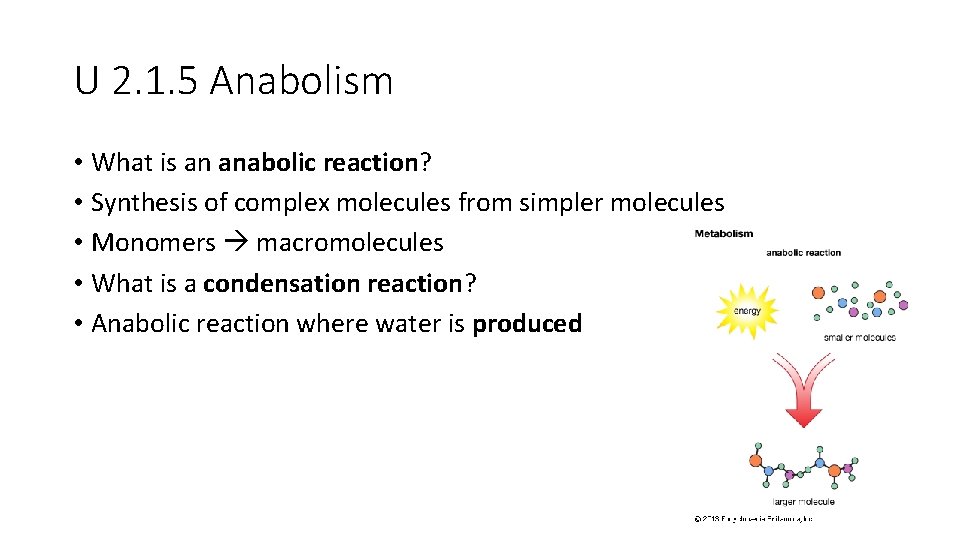
U 2. 1. 5 Anabolism • What is an anabolic reaction? • Synthesis of complex molecules from simpler molecules • Monomers macromolecules • What is a condensation reaction? • Anabolic reaction where water is produced
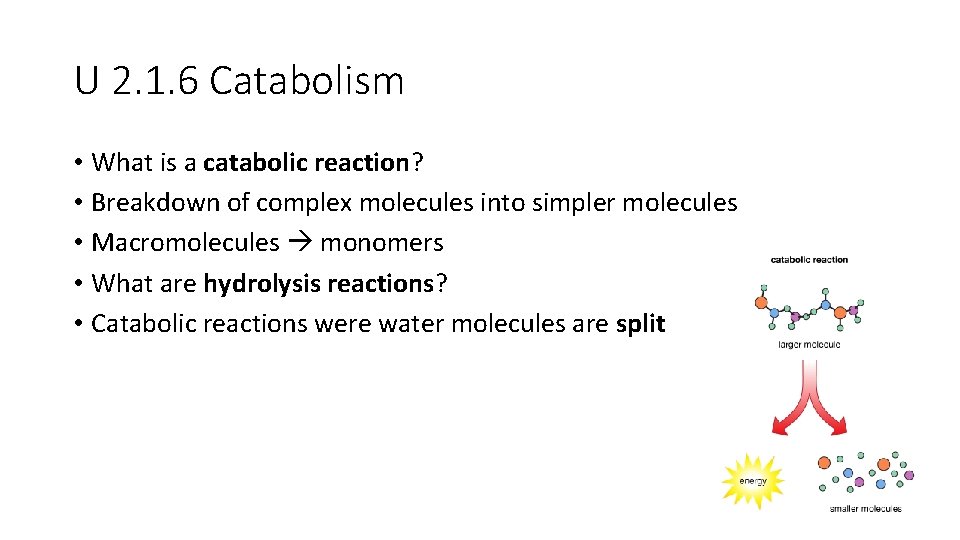
U 2. 1. 6 Catabolism • What is a catabolic reaction? • Breakdown of complex molecules into simpler molecules • Macromolecules monomers • What are hydrolysis reactions? • Catabolic reactions were water molecules are split
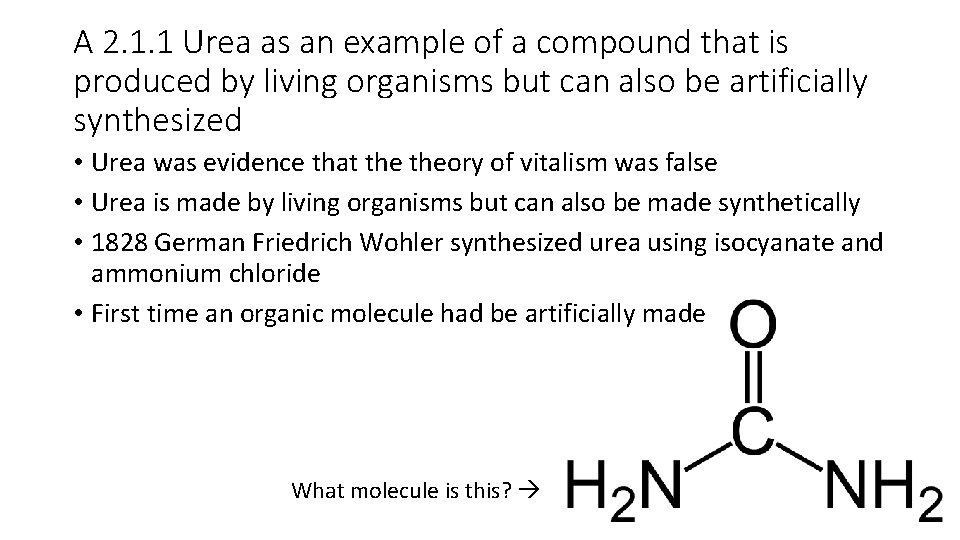
A 2. 1. 1 Urea as an example of a compound that is produced by living organisms but can also be artificially synthesized • Urea was evidence that theory of vitalism was false • Urea is made by living organisms but can also be made synthetically • 1828 German Friedrich Wohler synthesized urea using isocyanate and ammonium chloride • First time an organic molecule had be artificially made What molecule is this?
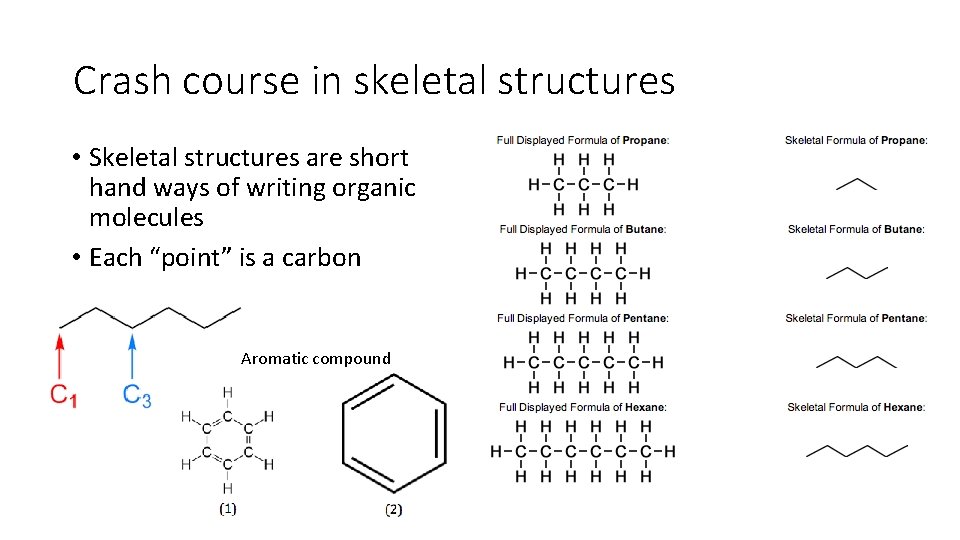
Crash course in skeletal structures • Skeletal structures are short hand ways of writing organic molecules • Each “point” is a carbon Aromatic compound
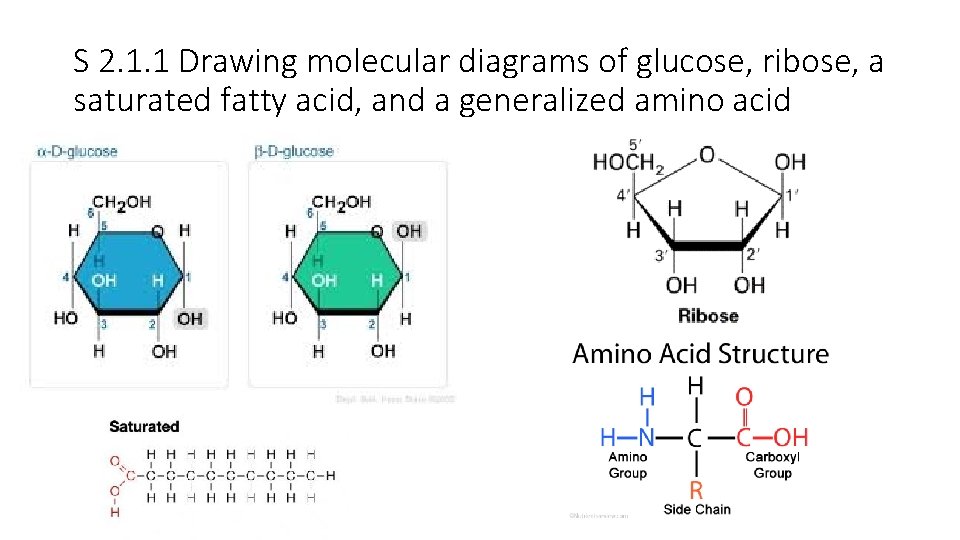
S 2. 1. 1 Drawing molecular diagrams of glucose, ribose, a saturated fatty acid, and a generalized amino acid
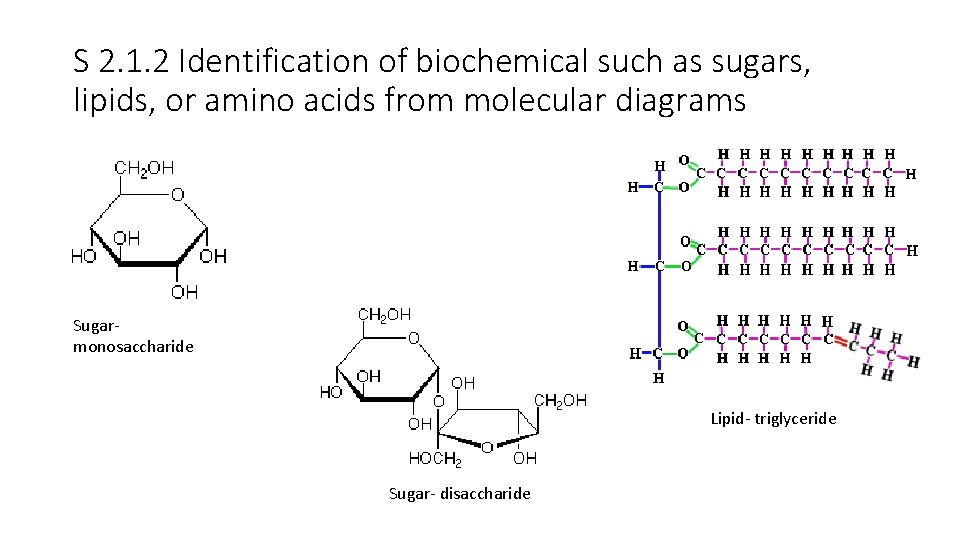
S 2. 1. 2 Identification of biochemical such as sugars, lipids, or amino acids from molecular diagrams Sugarmonosaccharide Lipid- triglyceride Sugar- disaccharide
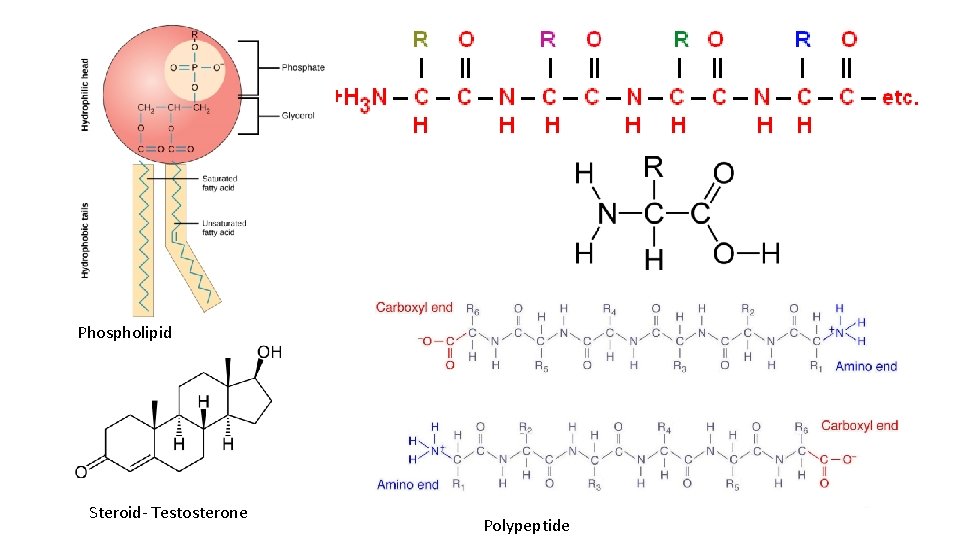
Phospholipid Steroid- Testosterone Polypeptide

Topic 2. 2 Water Essential idea: Water is the medium of life.

2. 2 Water Nature of science: Use theories to explain natural phenomena—the theory that hydrogen bonds form between water molecules explains the properties of water. (2. 2) Understandings • Water molecules are polar and hydrogen bonds form between them • Hydrogen bonding and dipolarity explain the cohesive, adhesive, thermal and solvent properties of water • Substance can be hydrophilic or hydrophobic Applications and Skills • Application: Comparison of thermal properties of water with those of methane • Application: Use of water as a coolant in sweat • Application: Modes of transport of glucose, amino acids, cholesterol, fats, oxygen, and sodium chloride in blood in relation to their solubility in water
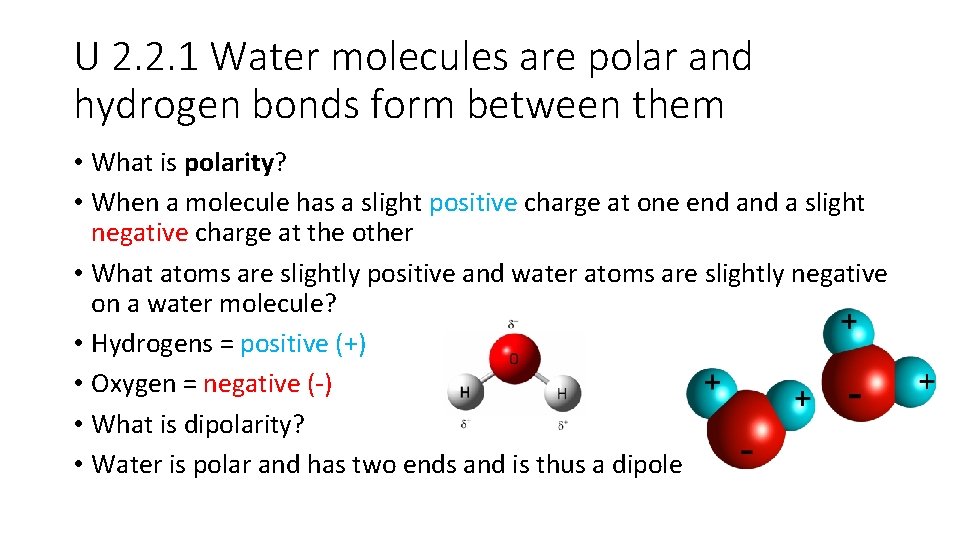
U 2. 2. 1 Water molecules are polar and hydrogen bonds form between them • What is polarity? • When a molecule has a slight positive charge at one end a slight negative charge at the other • What atoms are slightly positive and water atoms are slightly negative on a water molecule? • Hydrogens = positive (+) • Oxygen = negative (-) • What is dipolarity? • Water is polar and has two ends and is thus a dipole

U 2. 2. 1 Water molecules are polar and hydrogen bonds form between them • What are hydrogen bonds? • When a negative charge is attracted to the positive charge of hydrogen • So the negative charge of the oxygen atom can be attracted to the positive charge of the hydrogens in water • When hydrogen bonds are made is energy released or used? • Released • When hydrogen bonds break is energy released or used? • Used
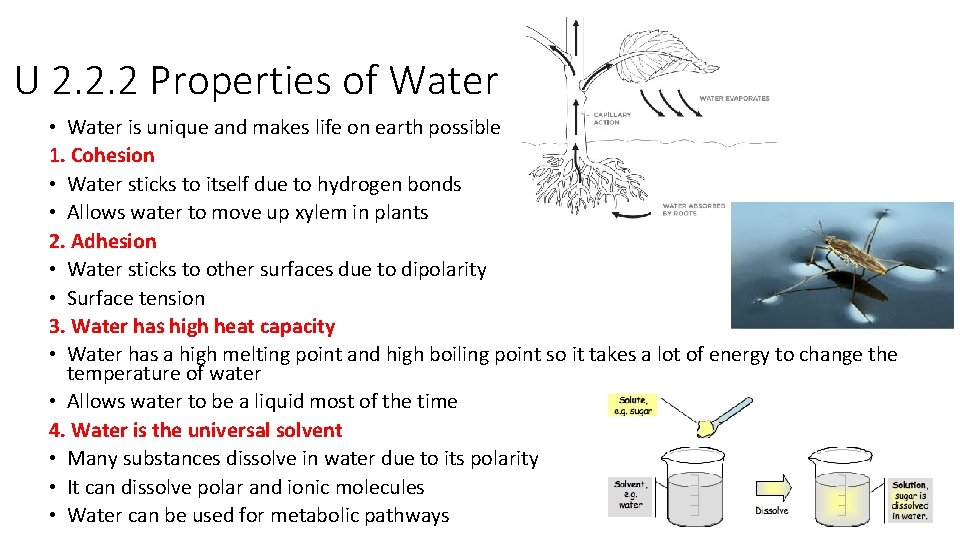
U 2. 2. 2 Properties of Water • Water is unique and makes life on earth possible 1. Cohesion • Water sticks to itself due to hydrogen bonds • Allows water to move up xylem in plants 2. Adhesion • Water sticks to other surfaces due to dipolarity • Surface tension 3. Water has high heat capacity • Water has a high melting point and high boiling point so it takes a lot of energy to change the temperature of water • Allows water to be a liquid most of the time 4. Water is the universal solvent • Many substances dissolve in water due to its polarity • It can dissolve polar and ionic molecules • Water can be used for metabolic pathways
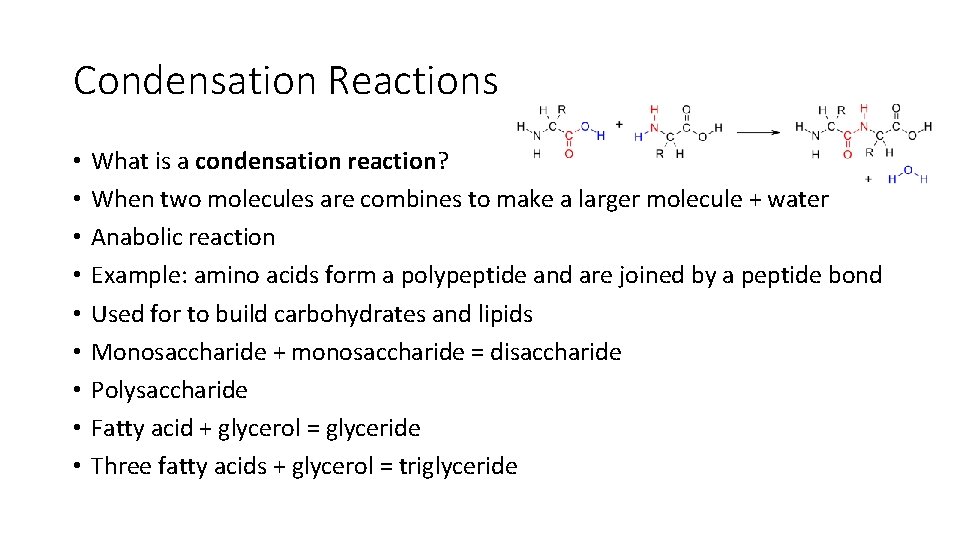
Condensation Reactions • • • What is a condensation reaction? When two molecules are combines to make a larger molecule + water Anabolic reaction Example: amino acids form a polypeptide and are joined by a peptide bond Used for to build carbohydrates and lipids Monosaccharide + monosaccharide = disaccharide Polysaccharide Fatty acid + glycerol = glyceride Three fatty acids + glycerol = triglyceride
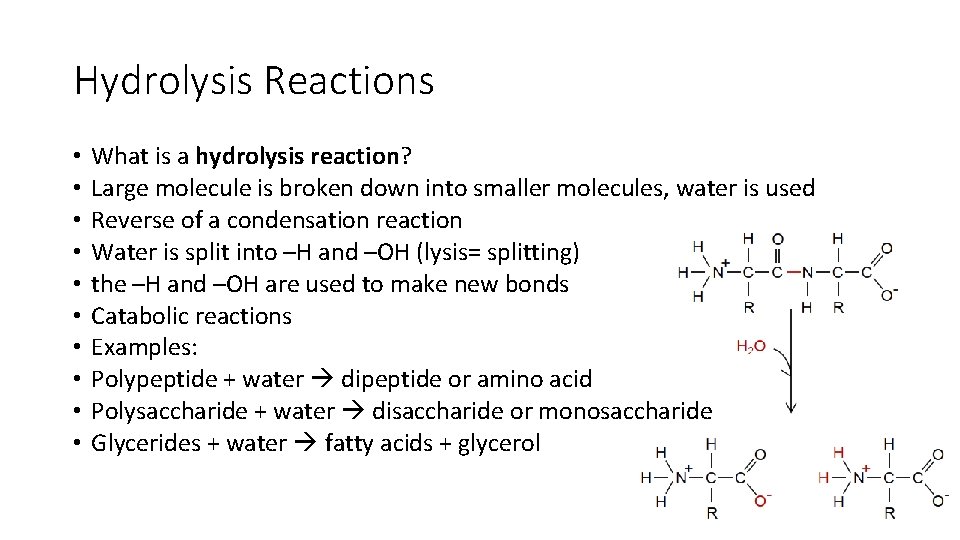
Hydrolysis Reactions • • • What is a hydrolysis reaction? Large molecule is broken down into smaller molecules, water is used Reverse of a condensation reaction Water is split into –H and –OH (lysis= splitting) the –H and –OH are used to make new bonds Catabolic reactions Examples: Polypeptide + water dipeptide or amino acid Polysaccharide + water disaccharide or monosaccharide Glycerides + water fatty acids + glycerol
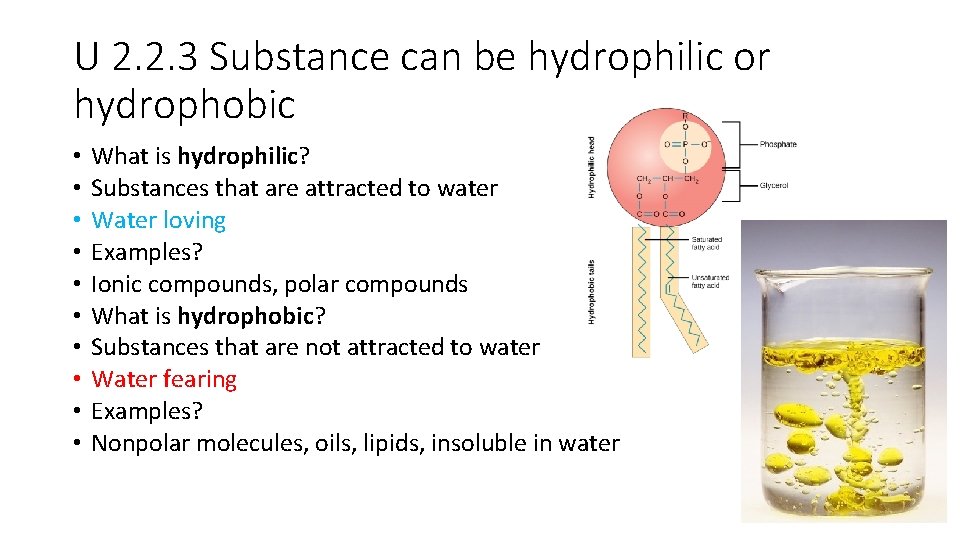
U 2. 2. 3 Substance can be hydrophilic or hydrophobic • • • What is hydrophilic? Substances that are attracted to water Water loving Examples? Ionic compounds, polar compounds What is hydrophobic? Substances that are not attracted to water Water fearing Examples? Nonpolar molecules, oils, lipids, insoluble in water
A 2. 2. 1 Comparison of thermal properties of water with those of methane • Purpose is to compare the importance of hydrogen bonding • Methane CH 4 has a similar molecular mass to water but has weaker intramolecular forces PROPERTY METHANE CH 4 WATER H 20 Melting Point -182⁰ C O⁰ C Boiling Point -160⁰ C 100⁰ C Specific Heat Capacity 2. 2 J/g/⁰C 4. 2 J/g/⁰C Latent Heat of Vaporization 760 J/g 2256 J/g • Hydrogen bonds restrict movement of water molecules so a lot more heat is needed to break melt ice • Water has a high heat capacity and a high heat of vaporization • A lot more energy is needed to break hydrogen bonds

A 2. 2. 2 Use of water as a coolant in sweat • When you exercise or do anything in high temperatures that increases body temperature you sweat • How is sweat a coolant? • When water evaporates hydrogen bonds are broken • Heat is needed to do this so when the sweat evaporate from the skin it removes the heat

A 2. 2. 3 Modes of transport of glucose, amino acids, cholesterol, fats, oxygen, and sodium chloride in blood in relation to their solubility in water 1. Glucose • Polar and can be dissolved in blood plasma 2. Amino acids • Polar and can be dissolved in blood plasma 3. Cholesterol • Non-polar and insoluble in water, transported as lipoproteins • Lipoprotein- small droplet, phospholipids and proteins coat the cholesterol molecule 4. Fats • Non-polar and insoluble in water, transported as lipoproteins 5. Oxygen • Non-polar and will bind to the hemoglobin in red blood cells 6. Sodium chloride • Soluble in water and is transported as Na+ and Cl- ions
- Slides: 24