Topic 12 The Periodic Table of Elements Valence

Topic #12: The Periodic Table of Elements

• Valence electrons - outermost electrons of an atom, which are important in determining how the atom reacts chemically with other atoms. • Ion-atom or molecule where the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. Cation – positively charged ion Anion – negatively charged ion • Oxidation Number-is equal to the charge on the ion
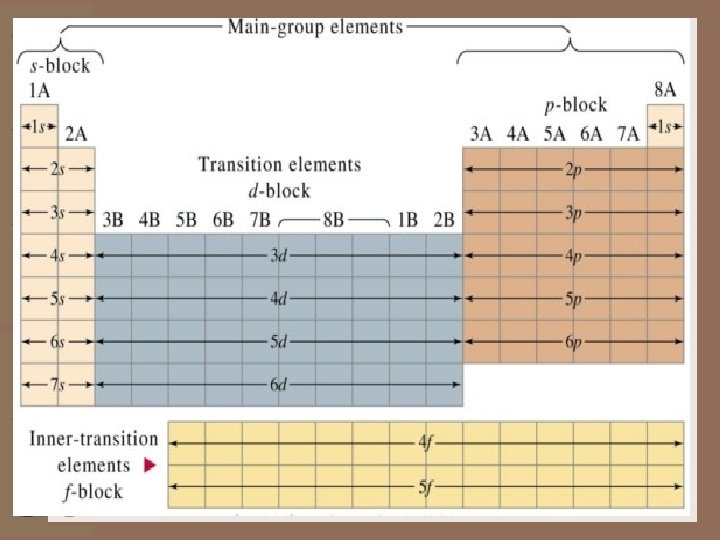

The Modern Periodic Table --- continued Period ( rows on the periodic table) The periodic table consists of rows called Periods (There a total of 7 rows or periods, beginning with hydrogen) • These rows also designate the energy level where electrons are found (more about this when we talk about electron configuration).
The Modern Periodic Table Groups (sometimes called Families) • are columns numbered from 1 -8, followed by a letter “A” or “B”. • The groups designated with a letter “A” (1 A to 8 A) are often referred as the main group or the “REPRESENTATIVE ELEMENTS”. • The groups designated with a “B” (1 B through 8 B) are referred to as the “TRANSITION ELEMENTS”. • A more recent numbering system, which uses the numbers 1 through 18, also appears above each group.
The Modern Periodic Table --Classifying the Elements Metals --- elements that are generally shiny when smooth and clean, solid at room temperature, good conductors of heat and electricity. • Most metals are ductile (drawn into wires) and malleable (moldable). Metals are in green
The Modern Periodic Table --Classifying the Elements Nonmetals --- elements that are generally gases or brittle, dull-looking solids. They are poor conductors of electricity. • The only nonmetal that is a liquid at room temperature is Bromine. Non metals are in orange
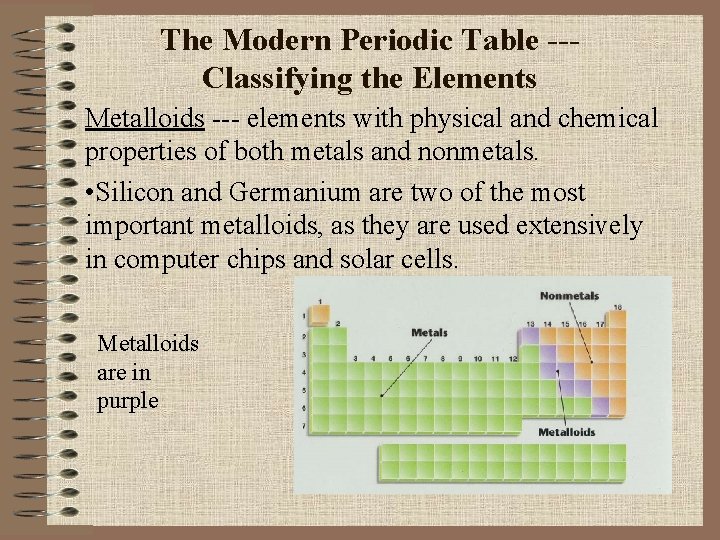
The Modern Periodic Table --Classifying the Elements Metalloids --- elements with physical and chemical properties of both metals and nonmetals. • Silicon and Germanium are two of the most important metalloids, as they are used extensively in computer chips and solar cells. Metalloids are in purple

THE REPRESENTATIVE ELEMENTS Group 1 A – The Alkali Metals • Elements in this family have 1 valence electron. • Lose their valence electron to form a +1 charged ion. • Belong to the s-block in the periodic table.
THE REPRESENTATIVE ELEMENTS Group 2 A – The Alkaline Earth Metals • Elements in this family have 2 valence electrons. • Lose their valence electrons to form a +2 charged ion. • Belong to the s-block in the periodic table.
THE REPRESENTATIVE ELEMENTS Group 3 A – The Boron Group • Is named for the metalloid Boron • Elements in this family have 3 valence electrons • Lose their valence electrons to form a +3 charged ion. • Belong to the p-block in the periodic table
THE REPRESENTATIVE ELEMENTS Group 4 A – The Carbon Group • Is named for the nonmetal Carbon • Elements in this family have 4 valence electrons • Lose their valence electrons to form a +4 charged ion OR can gain 4 electrons and form a – 4 charged ion. • Belong to the p-block in the periodic table.
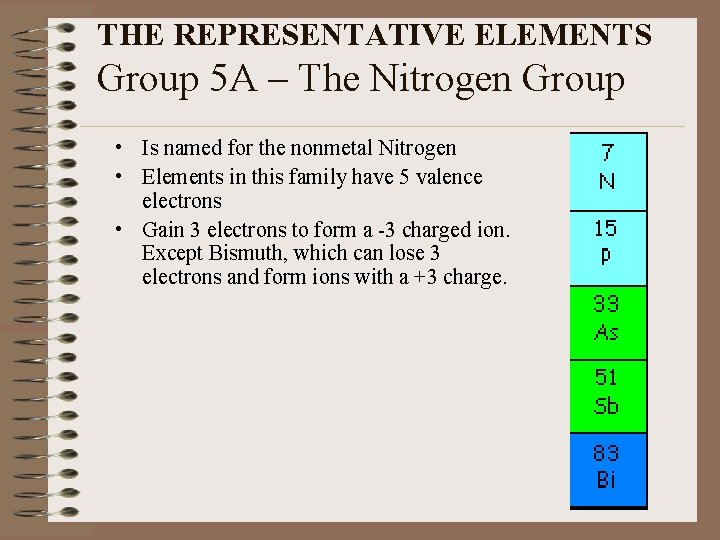
THE REPRESENTATIVE ELEMENTS Group 5 A – The Nitrogen Group • Is named for the nonmetal Nitrogen • Elements in this family have 5 valence electrons • Gain 3 electrons to form a -3 charged ion. Except Bismuth, which can lose 3 electrons and form ions with a +3 charge.
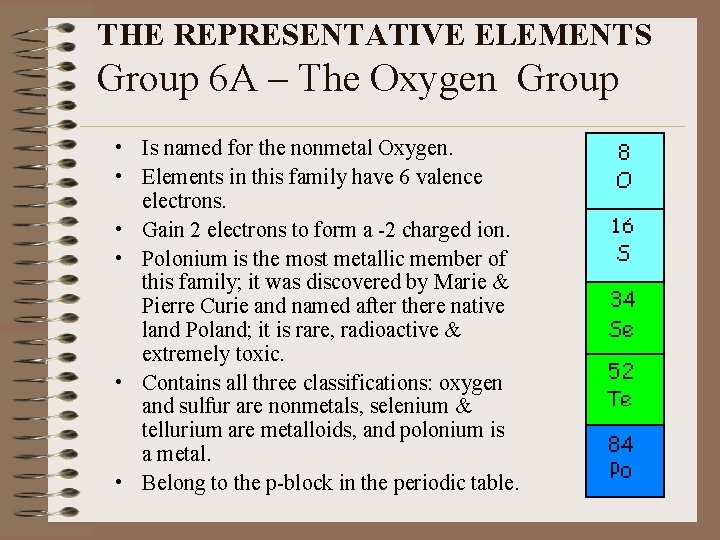
THE REPRESENTATIVE ELEMENTS Group 6 A – The Oxygen Group • Is named for the nonmetal Oxygen. • Elements in this family have 6 valence electrons. • Gain 2 electrons to form a -2 charged ion. • Polonium is the most metallic member of this family; it was discovered by Marie & Pierre Curie and named after there native land Poland; it is rare, radioactive & extremely toxic. • Contains all three classifications: oxygen and sulfur are nonmetals, selenium & tellurium are metalloids, and polonium is a metal. • Belong to the p-block in the periodic table.
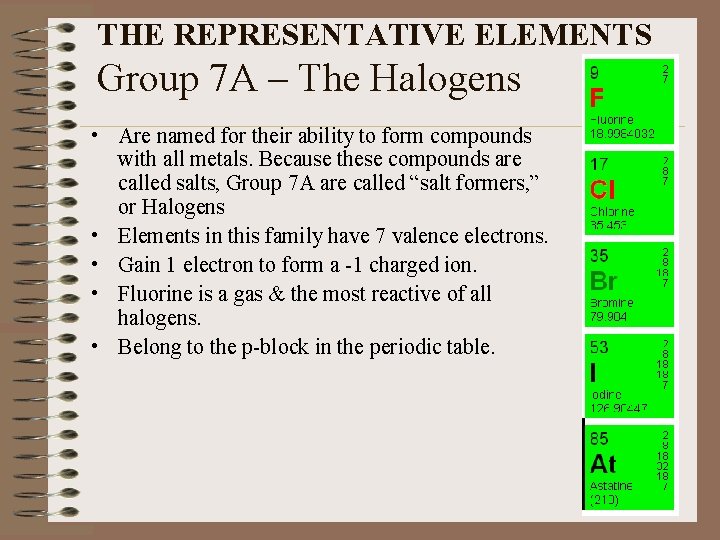
THE REPRESENTATIVE ELEMENTS Group 7 A – The Halogens • Are named for their ability to form compounds with all metals. Because these compounds are called salts, Group 7 A are called “salt formers, ” or Halogens • Elements in this family have 7 valence electrons. • Gain 1 electron to form a -1 charged ion. • Fluorine is a gas & the most reactive of all halogens. • Belong to the p-block in the periodic table.

THE REPRESENTATIVE ELEMENTS Group 8 A – The Noble Gases • Were among the last naturally occurring elements to be discovered because they are colorless and unreactive. • Elements in this family have 8 valence electrons. This means they have the maximum number of electrons in their outermost energy level, 8, except for helium, which has two. • Belong to the p-block in the periodic table.

THE TRANSITION METALS Groups 1 B- 8 B (or more commonly referred to as Groups 3 -12) • Most are hard solids with high melting & boiling points. • Transition metals can lose 2 s electrons and form an ion with a +2 charge • Because unpaired electrons can move to the outer energy level, these elements can form ions with a +3 charge or higher, as well. • Transition metals belong to the d-block in the periodic table
THE INNER TRANSITION METALS -- The Lanthanide Series (Period 6 on the Periodic Table) • Silvery metals with relatively high melting points. • Because there is very little variation in properties among the inner transition metals, they are found mixed together in nature and are extremely difficult to separate. • Belong to the f-block in the periodic table
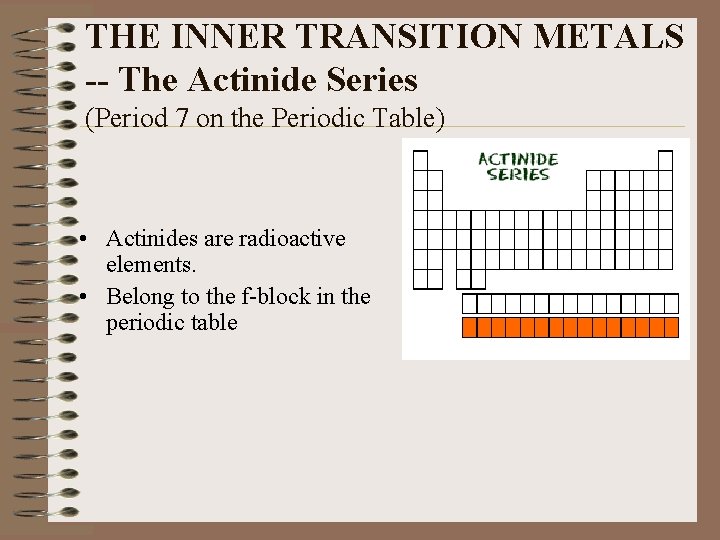
THE INNER TRANSITION METALS -- The Actinide Series (Period 7 on the Periodic Table) • Actinides are radioactive elements. • Belong to the f-block in the periodic table
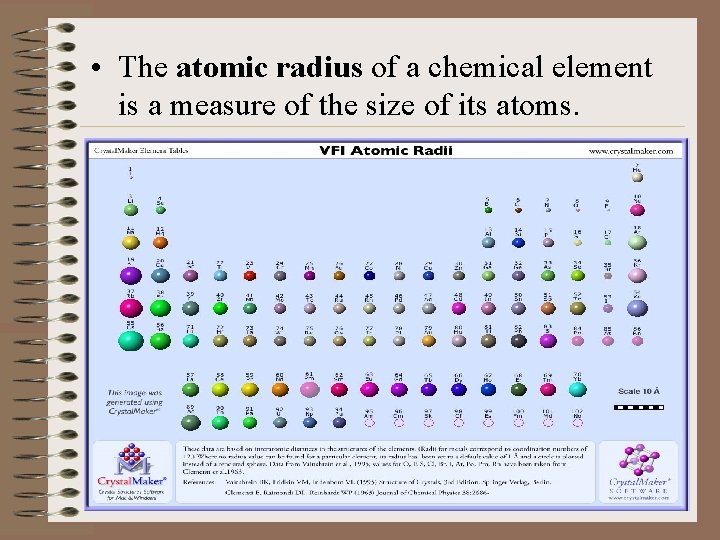
• The atomic radius of a chemical element is a measure of the size of its atoms.
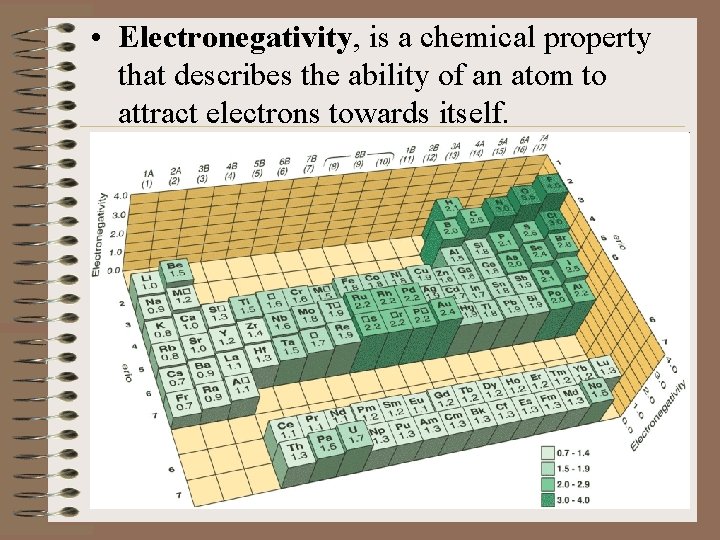
• Electronegativity, is a chemical property that describes the ability of an atom to attract electrons towards itself.
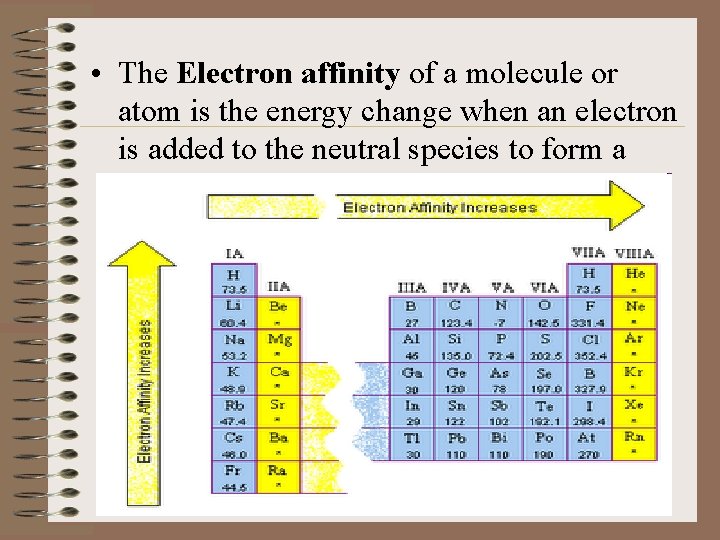
• The Electron affinity of a molecule or atom is the energy change when an electron is added to the neutral species to form a negative ion.
• The ionization energy (EI) of an atom is the minimal energy required to remove electrons from atoms.
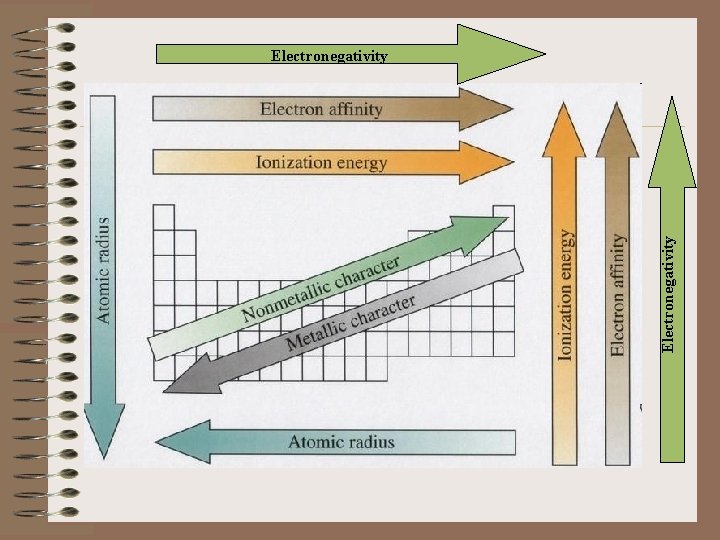
Electronegativity
- Slides: 24