Topic 11 Rocks and Minerals Minerals are economically























- Slides: 30

Topic 11 Rocks and Minerals

Minerals are economically important http: //www. mii. org/

Minerals • • Natural Solid Inorganic Definite chemical composition • Crystal structure due to internal arrangement of atoms

• There about 3000 known minerals • Minerals are made of elements (either a single element or a combination of elements) • Examples of Minerals – Native elements such as gold, a mineral made of one element (gold…Au) – Compounds such as calcite, a mineral made of 3 elements (calcium, carbon, and oxygen…Ca. CO 3) gold calcite


Less than a dozen are common in most rocks • Quartz • Feldspar (group) • Muscovite (white mica) • Biotite (black mica) • Calcite • Pyroxene • Olivine • Amphibole (group) • Magnetite, limonite, and other iron oxides • Pyrite

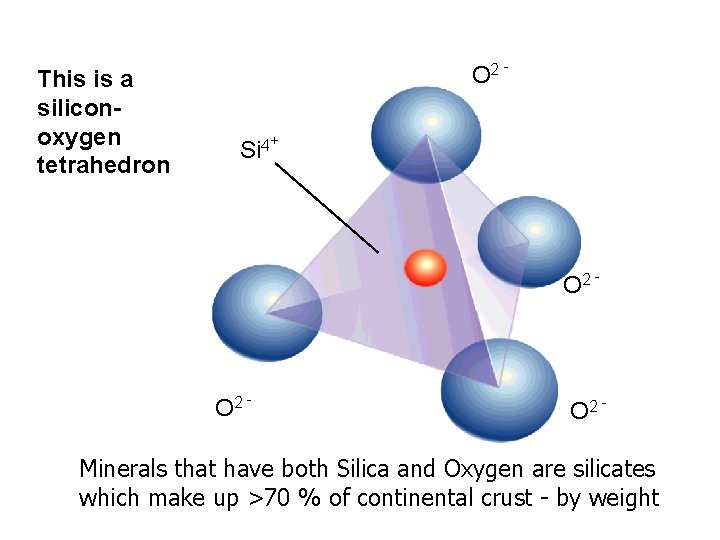
This is a siliconoxygen tetrahedron O 2 Si 4+ O 2 - Minerals that have both Silica and Oxygen are silicates which make up >70 % of continental crust - by weight

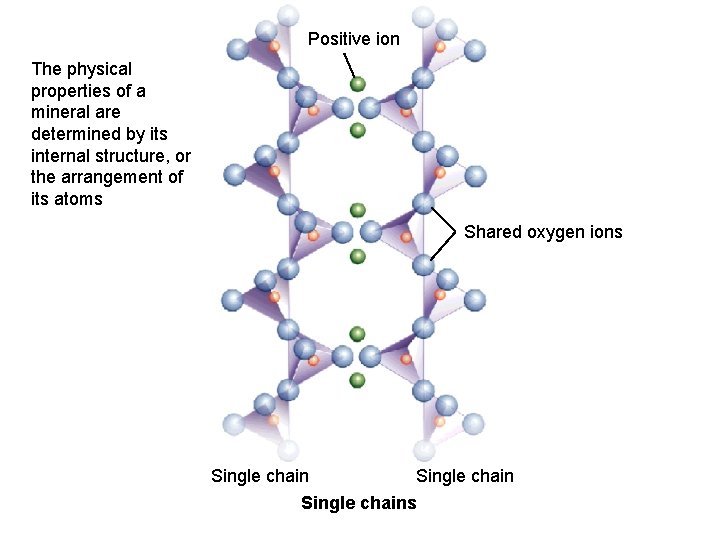
Positive ion The physical properties of a mineral are determined by its internal structure, or the arrangement of its atoms 2_26 b Shared oxygen ions Single chains

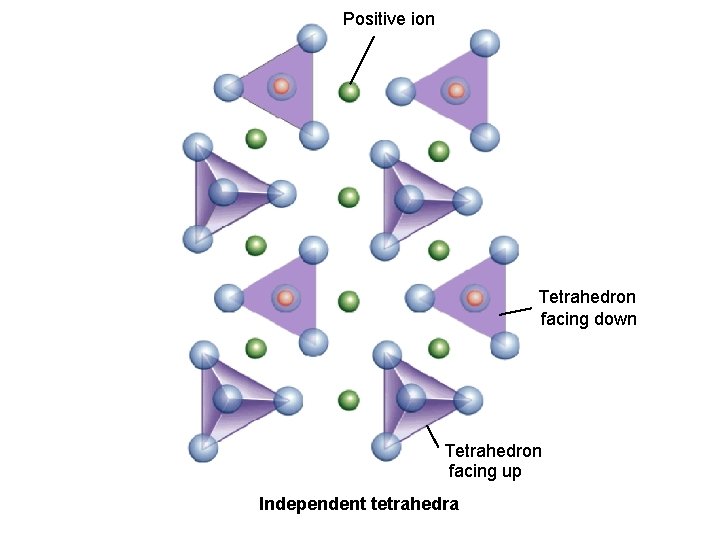
Positive ion 2_26 a Tetrahedron facing down Tetrahedron facing up Independent tetrahedra

The physical properties of a mineral are determined by its internal structure, or the arrangement of its atoms Silicate Minerals


Minerals are identified by their key characteristics • hardness • crystal shape (form) • luster • color • streak • cleavage/fracture • density (specific gravity) • special properties -reaction to acid -fluorescence -salty taste -magnetism

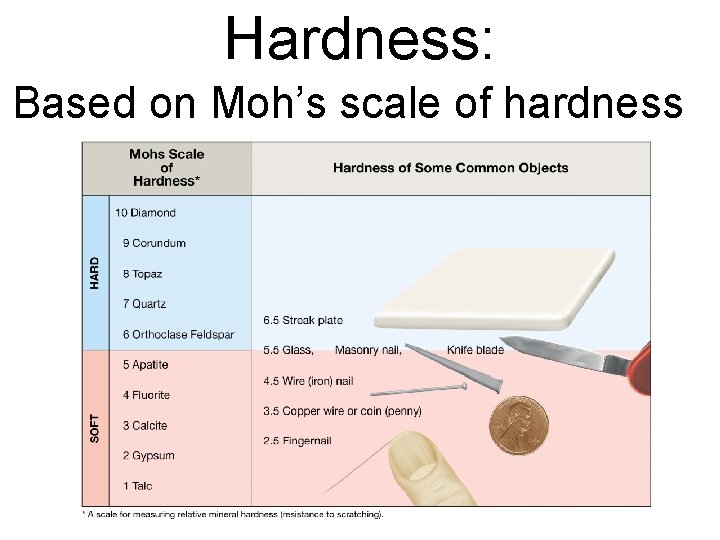
Mineral Hardness • Ability to scratch another mineral • Mohs scale from 1 (talc) to 10 (diamond) • Quartz (most common mineral and most dust particles) is 7

Mohs Mineral Hardness Scale 1) Talc Softest 2) Gypsum 3) Calcite 1 5 4) Flourite 5) Apatite 2 6) Feldspar 6 7) Quartz 8) Topaz 3 7 9) Corundum 10) Diamond 9 Hardest 4 8 10

Hardness: Based on Moh’s scale of hardness

Crystal Shape The shape a mineral takes if grown unimpeded Mineral Java Applet

Luster • Describes how light reflects off the surface • Main categories are “metallic” and “nonmetallic” • Non-metallic includes “dull, ” glassy, ” waxy, ” “pearly, ” and others

Luster: how a mineral reflects light Metallic Non-metallic

Color • results from ability to absorb some wavelengths and reflect others • some minerals have characteristic colors • others vary due to chemical differences or impurities (atoms mixed inside the main elements) • Color is not reliable

Streak • Color of the powder when rubbed on a “streak plate” (unglazed porcelain) • May be same as hand-specimen or different • Some paint is based on powdered minerals (streaks).

Streak: The powdered form of a mineral

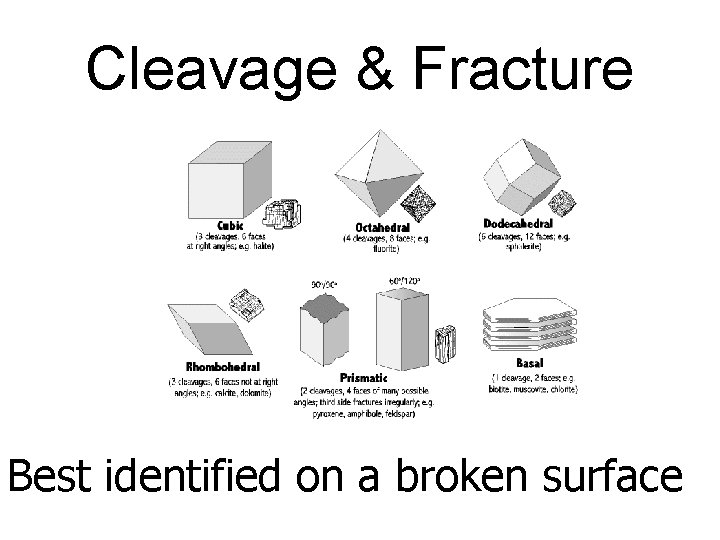
Mineral cleavage/fracture • Some minerals split along flat surfaces when struck hard--this is called mineral cleavage • Other minerals break unevenly along rough or curved surfaces--this is called fracture

Cleavage & Fracture Best identified on a broken surface

Density (Specific Gravity) • All minerals have density (mass / volume), but some are very dense • Examples include galena, magnetite, and gold • Specific Gravity is the density of the mineral compared with density of water

Special Characteristics-the “Acid Test” Carbonates react with dilute HCl and other acids by fizzing or bubbling (releasing CO 2 gas)

Special Characteristics-Salty Taste • DO NOT TASTE MOST MINERALS! • Halite is the exception --it will taste salty

Special Characteristics-Magnetism • Many iron minerals will produce an invisible magnetic force field • “Lodestone” was used by Vikings more than 1, 000 years ago as compasses

Mineral Formation Minerals form 2 ways: 1. solidification of magma 2. precipitation of ions as water evaporates


That’s a lot of salt!!!!
 Antigentest åre
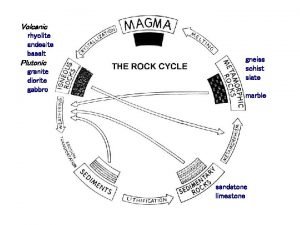
Antigentest åre Types of igneous sedimentary and metamorphic rocks
Types of igneous sedimentary and metamorphic rocks Igneous metamorphic sedimentary
Igneous metamorphic sedimentary Cementation rocks
Cementation rocks Granite and basalt difference
Granite and basalt difference Difference between minerals and rocks
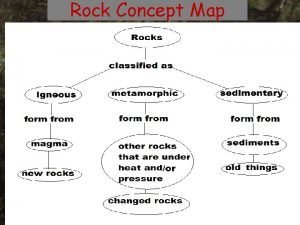
Difference between minerals and rocks Concept map of classification of rocks
Concept map of classification of rocks Poem about minerals and rocks 3 stanza
Poem about minerals and rocks 3 stanza What kind of rock is this
What kind of rock is this Rocks and minerals
Rocks and minerals Big problemo
Big problemo Extrusive vs intrusive igneous rocks
Extrusive vs intrusive igneous rocks Naviance lasa
Naviance lasa Quartzite rock cycle
Quartzite rock cycle Rocks are aggregates of minerals
Rocks are aggregates of minerals Clincher sentence examples
Clincher sentence examples Narrow
Narrow Minerals and their functions sources and deficiency chart
Minerals and their functions sources and deficiency chart Chapter 8 vitamins and minerals
Chapter 8 vitamins and minerals What are the elements of major and minor minerals
What are the elements of major and minor minerals Absorbs water and minerals
Absorbs water and minerals Padma mines and minerals corporation
Padma mines and minerals corporation Stores minerals and anchors muscles
Stores minerals and anchors muscles Cumbria minerals and waste local plan
Cumbria minerals and waste local plan What are resources that can be replaced
What are resources that can be replaced Minerals and fuels
Minerals and fuels Deficiency chart of macronutrients
Deficiency chart of macronutrients Minerals and fuels
Minerals and fuels Importance of rocks
Importance of rocks Flow chart of vitamins
Flow chart of vitamins Uniaxial indicatrix
Uniaxial indicatrix