Tools of Biology Dissecting Microscope Compound Microscope Electron
Tools of Biology
Dissecting Microscope Compound Microscope Electron Microscope TOOLS FOR DETECTION OF MICROSCOPIC OBJECTS
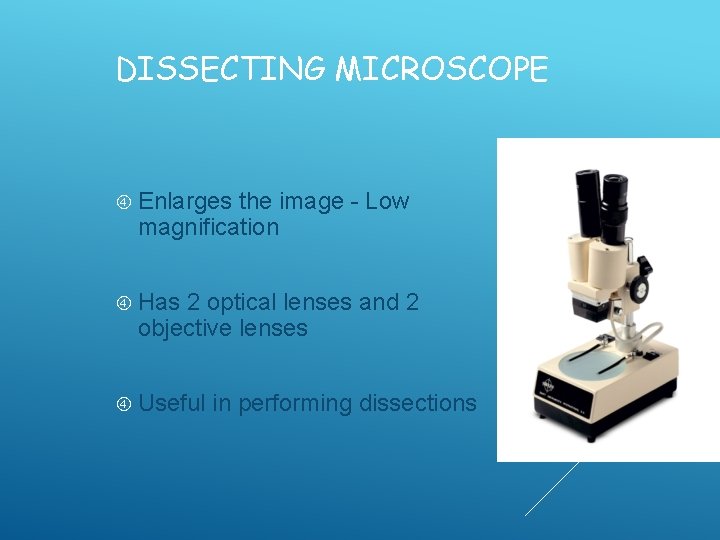
DISSECTING MICROSCOPE Enlarges the image - Low magnification Has 2 optical lenses and 2 objective lenses Useful in performing dissections

COMPOUND MICROSCOPE Used to observe small objects on a slide Specimen must be thin enough to let light pass through Has 1 optical lens and 2 or 3 objective lenses When looking through microscope the image will appear: Enlarged Reversed Flipped upside down

ELECTRON MICROSCOPE Magnifies up to 10, 000 x The light source is replaced by a beam of very fast moving electrons. The specimen usually has to be specially prepared and held inside a vacuum chamber from which the air has been pumped out (because electrons do not travel very far in air). The lenses are replaced by a series of coil-shaped electromagnets through which the electron beam travels. In an ordinary microscope, the glass lenses bend (or refract) the light beams passing through them to produce magnification. In an electron microscope, the coils bend the electron beams the same way. The image is formed as a photograph (called an electron micrograph) or as an image on a TV screen.
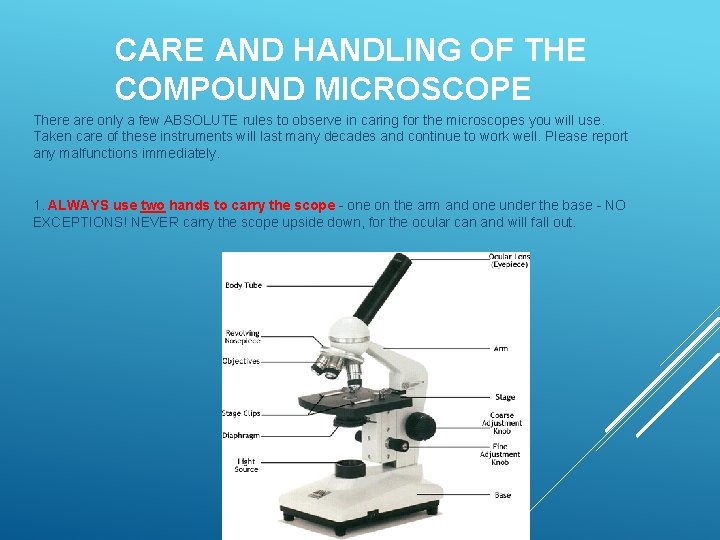
CARE AND HANDLING OF THE COMPOUND MICROSCOPE There are only a few ABSOLUTE rules to observe in caring for the microscopes you will use. Taken care of these instruments will last many decades and continue to work well. Please report any malfunctions immediately. 1. ALWAYS use two hands to carry the scope - one on the arm and one under the base - NO EXCEPTIONS! NEVER carry the scope upside down, for the ocular can and will fall out.

2. Always use the proper focusing technique to avoid ramming the objective lens into a slide - this can break the objective lens and/or ruin an expensive slide. *Do not use course adjustment knob when using high power objective lens!**

3. When finished using the microscope… …Always turn off the light and move objective lens back to low power

LETS GET A MICROSCOPE!!
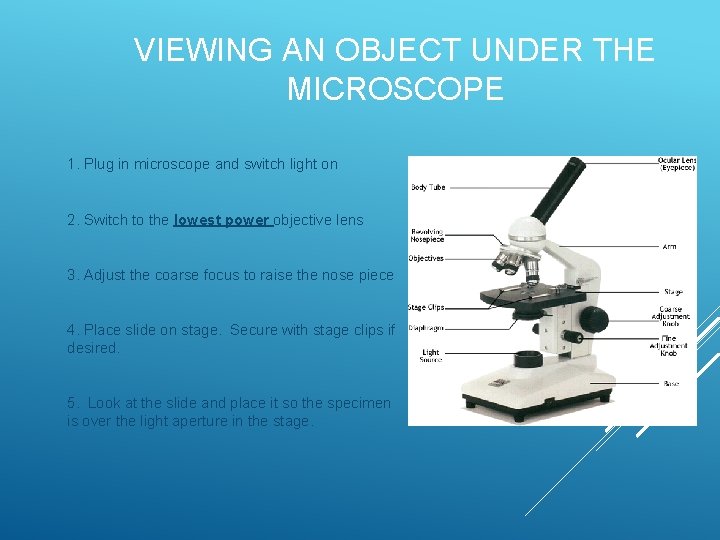
VIEWING AN OBJECT UNDER THE MICROSCOPE 1. Plug in microscope and switch light on 2. Switch to the lowest power objective lens 3. Adjust the coarse focus to raise the nose piece 4. Place slide on stage. Secure with stage clips if desired. 5. Look at the slide and place it so the specimen is over the light aperture in the stage.
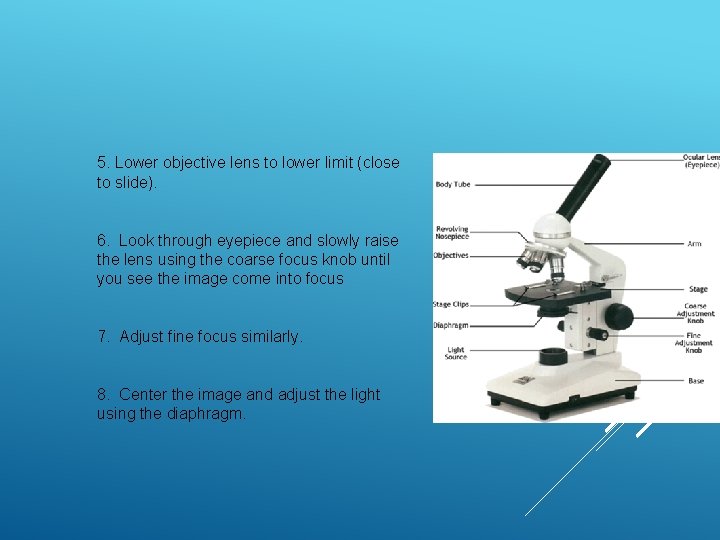
5. Lower objective lens to lower limit (close to slide). 6. Look through eyepiece and slowly raise the lens using the coarse focus knob until you see the image come into focus 7. Adjust fine focus similarly. 8. Center the image and adjust the light using the diaphragm.
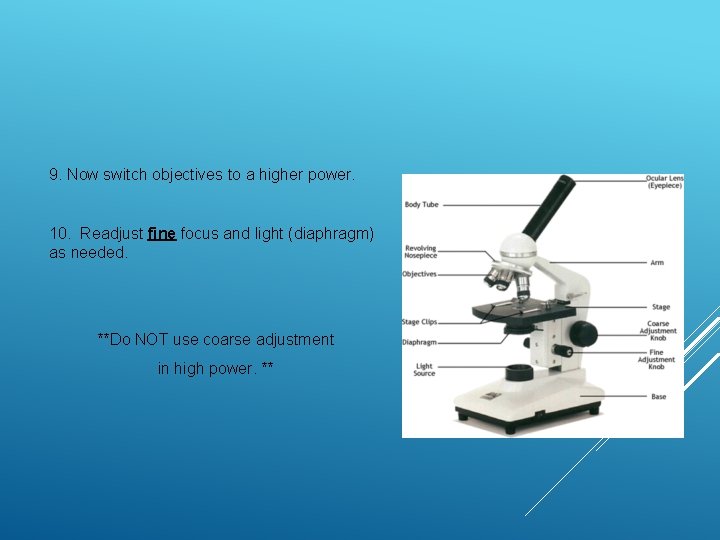
9. Now switch objectives to a higher power. 10. Readjust fine focus and light (diaphragm) as needed. **Do NOT use coarse adjustment in high power. **

START HERE PER 2/3


Things To Remember! All microscopes: ◦ Enlarge ◦ Reverse ◦ Invert For example: EIHS
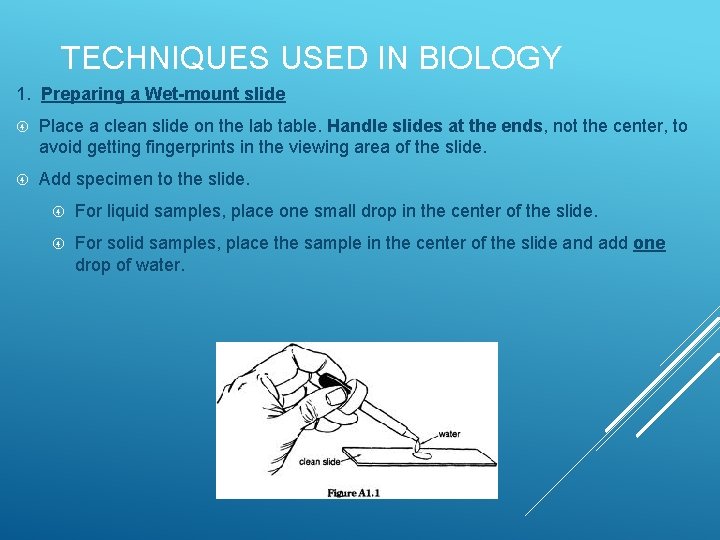
TECHNIQUES USED IN BIOLOGY 1. Preparing a Wet-mount slide Place a clean slide on the lab table. Handle slides at the ends, not the center, to avoid getting fingerprints in the viewing area of the slide. Add specimen to the slide. For liquid samples, place one small drop in the center of the slide. For solid samples, place the sample in the center of the slide and add one drop of water.
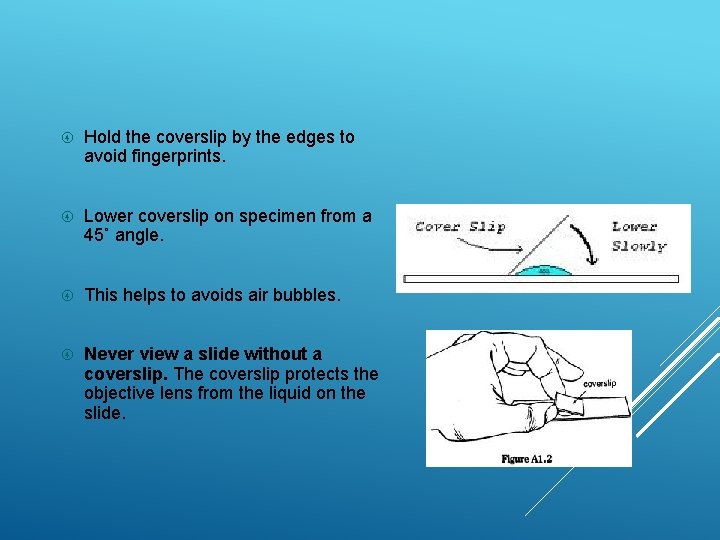
Hold the coverslip by the edges to avoid fingerprints. Lower coverslip on specimen from a 45˚ angle. This helps to avoids air bubbles. Never view a slide without a coverslip. The coverslip protects the objective lens from the liquid on the slide.
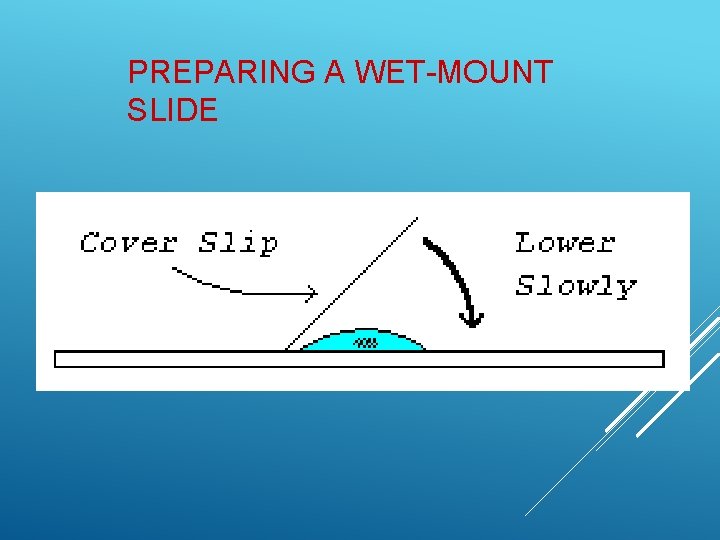
PREPARING A WET-MOUNT SLIDE
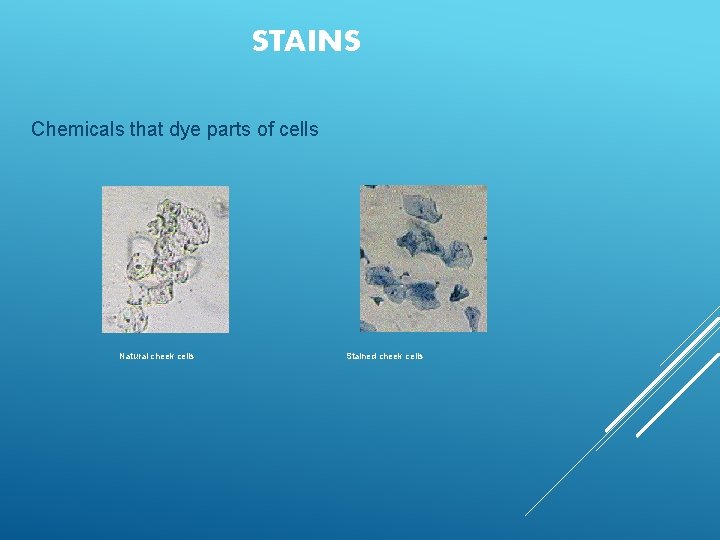
STAINS Chemicals that dye parts of cells Natural cheek cells Stained cheek cells
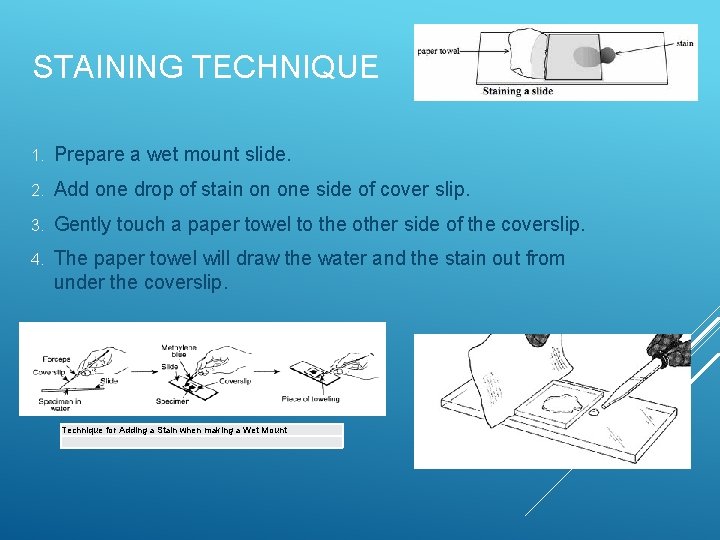
STAINING TECHNIQUE 1. Prepare a wet mount slide. 2. Add one drop of stain on one side of cover slip. 3. Gently touch a paper towel to the other side of the coverslip. 4. The paper towel will draw the water and the stain out from under the coverslip. Technique for Adding a Stain when making a Wet Mount
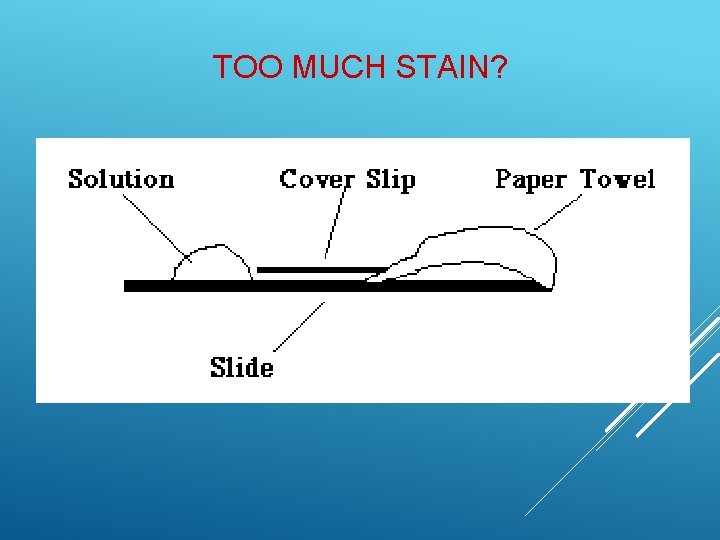
TOO MUCH STAIN?

1. LUGOL'S IODINE SOLUTION Commonly used to stain plant cells – turns dark in the presence of starch and cell walls contain a lot of starch
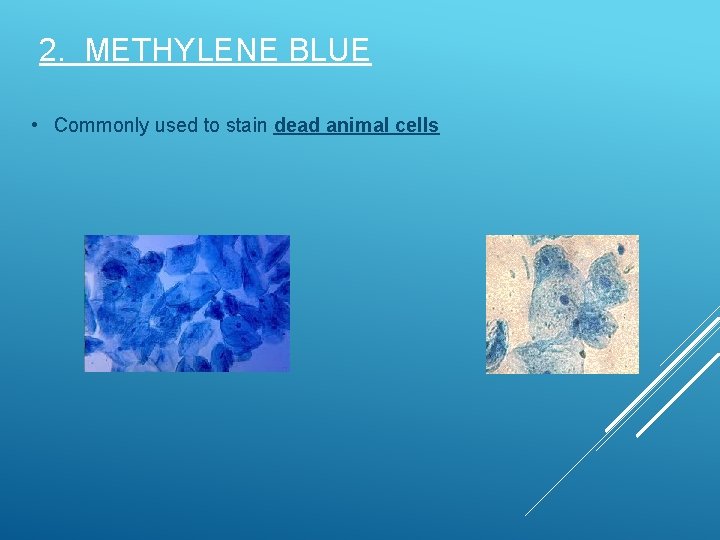
2. METHYLENE BLUE • Commonly used to stain dead animal cells

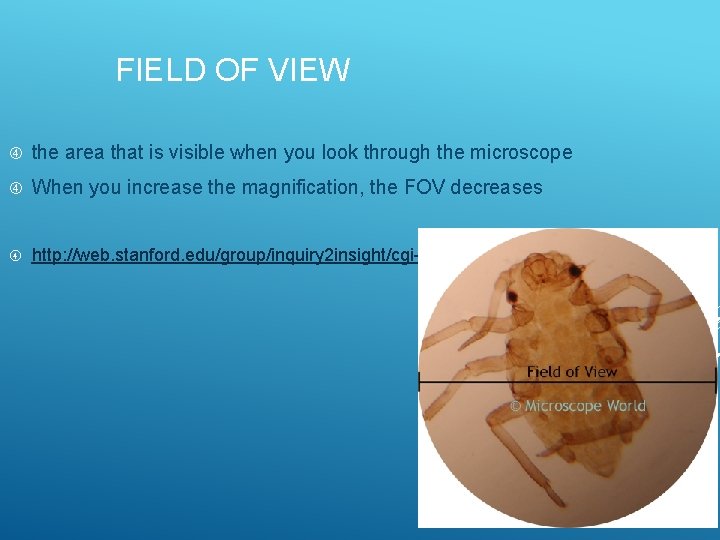
FIELD OF VIEW the area that is visible when you look through the microscope When you increase the magnification, the FOV decreases http: //web. stanford. edu/group/inquiry 2 insight/cgi-bin/vu-r 1 a/vu. php? view=micmeas

ESTIMATING THE SIZE OF AN OBJECT UNDER THE MICROSCOPE Diameter of the FOV Estimated size = Number of objects that fit across
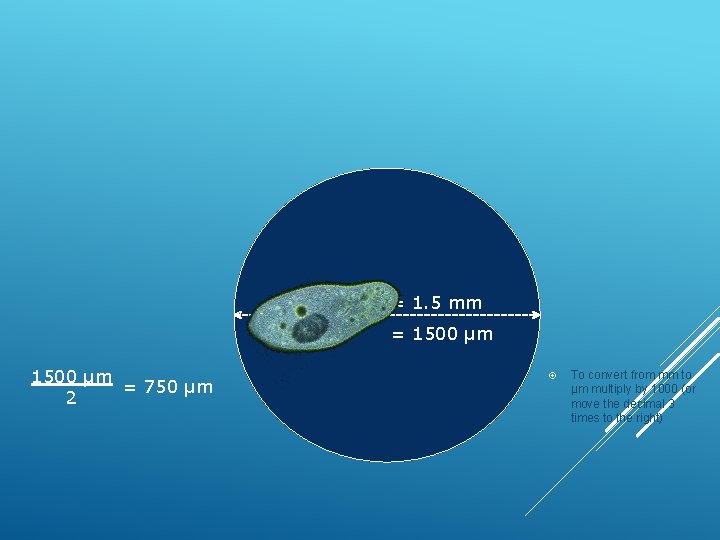
Diameter = 1. 5 mm = 1500 µm = 750 µm 2 To convert from mm to µm multiply by 1000 (or move the decimal 3 times to the right)

OTHER TOOLS IN BIOLOGY
DETECTING SUBSTANCES 1. Indicators show a reaction (usually a color change) to signify the presence of a substance Many indicators are used to test p. H level
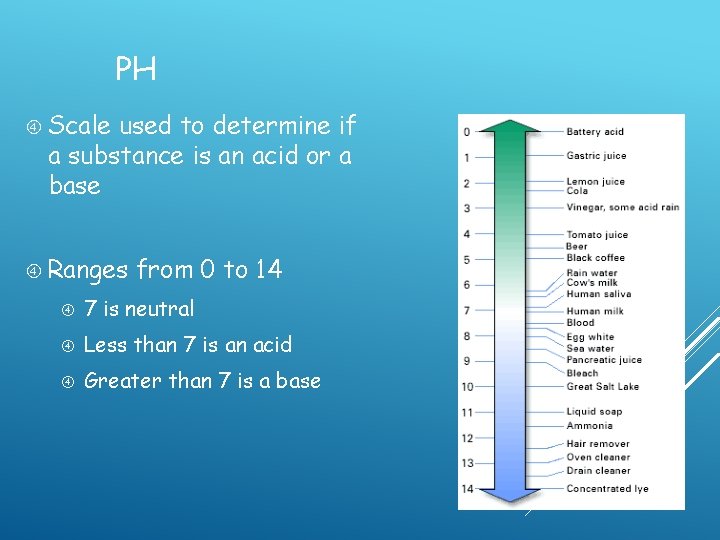
PH Scale used to determine if a substance is an acid or a base Ranges from 0 to 14 7 is neutral Less than 7 is an acid Greater than 7 is a base
TESTING PH Litmus paper - contains a substance that detects the presence of acids or bases
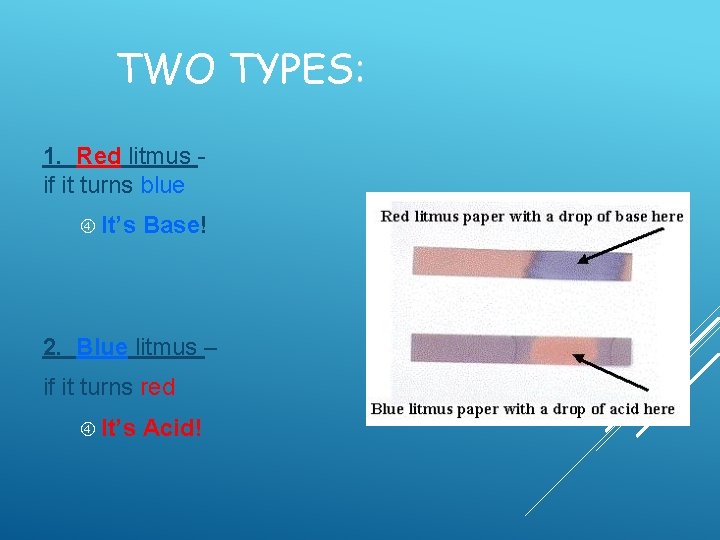
TWO TYPES: 1. Red litmus if it turns blue It’s Base! 2. Blue litmus – if it turns red It’s Acid!
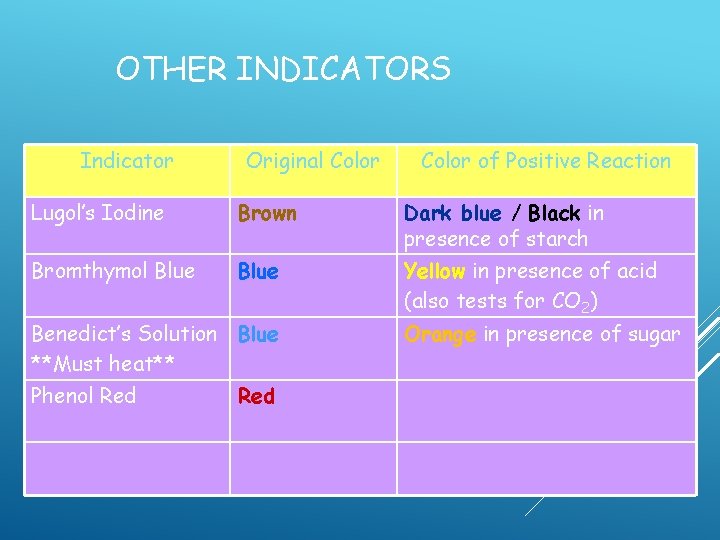
OTHER INDICATORS Indicator Original Color of Positive Reaction
OTHER INDICATORS Indicator Lugol’s Iodine Original Color of Positive Reaction
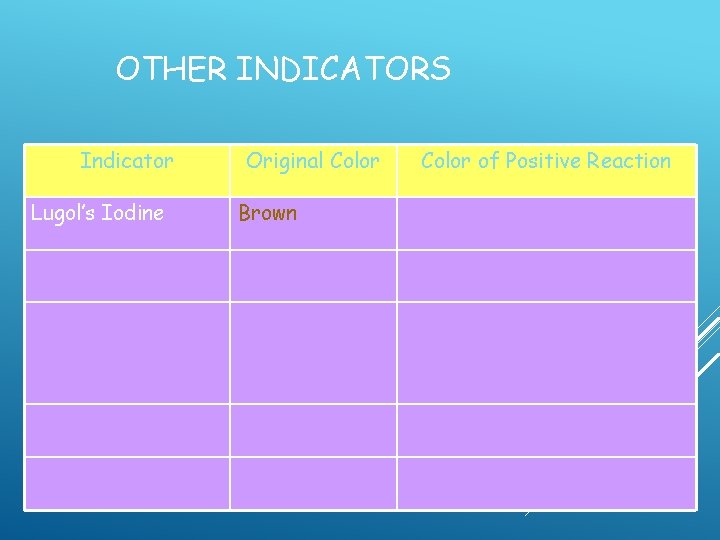
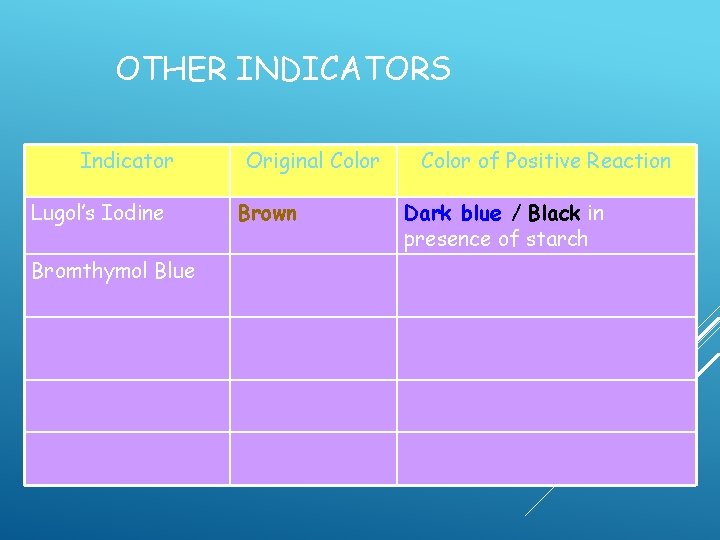
OTHER INDICATORS Indicator Lugol’s Iodine Original Color Brown Color of Positive Reaction
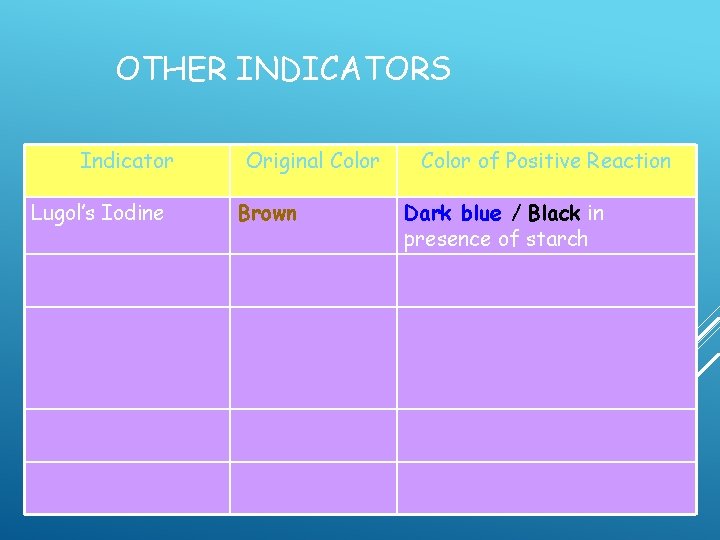
OTHER INDICATORS Indicator Lugol’s Iodine Original Color Brown Color of Positive Reaction Dark blue / Black in presence of starch
OTHER INDICATORS Indicator Lugol’s Iodine Bromthymol Blue Original Color Brown Color of Positive Reaction Dark blue / Black in presence of starch
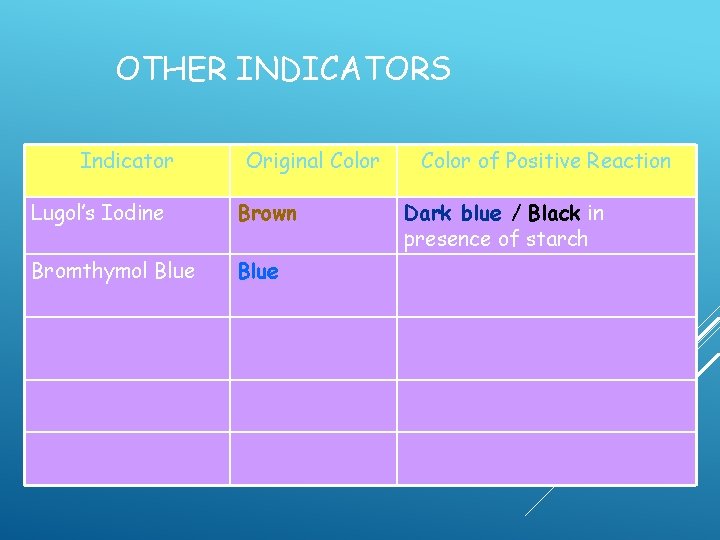
OTHER INDICATORS Indicator Original Color Lugol’s Iodine Brown Bromthymol Blue Color of Positive Reaction Dark blue / Black in presence of starch
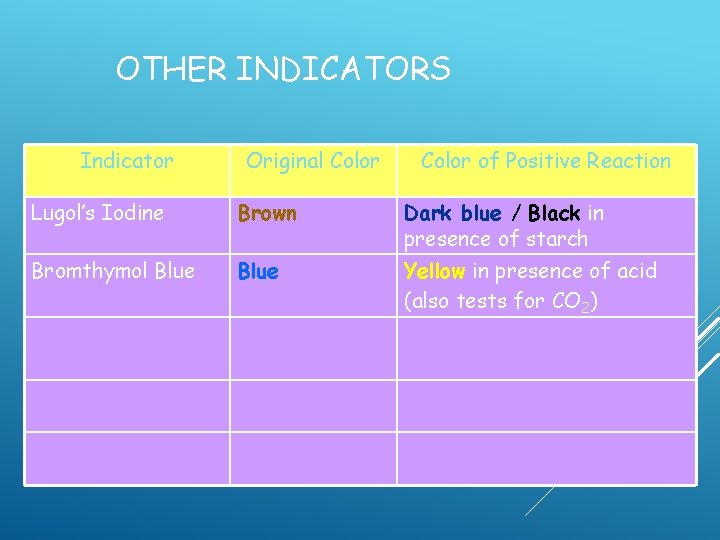
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2)
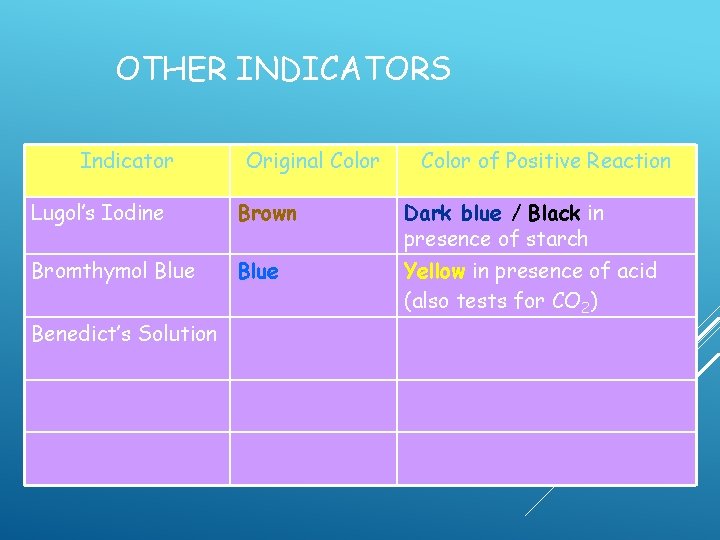
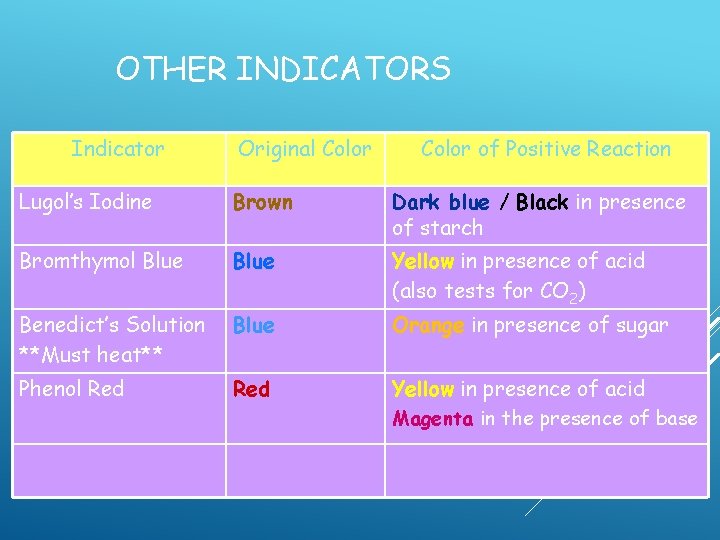
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution

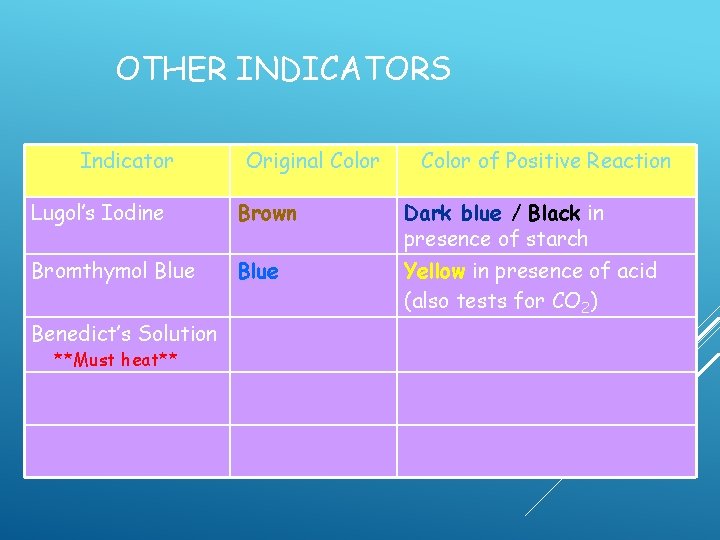
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution **Must heat**
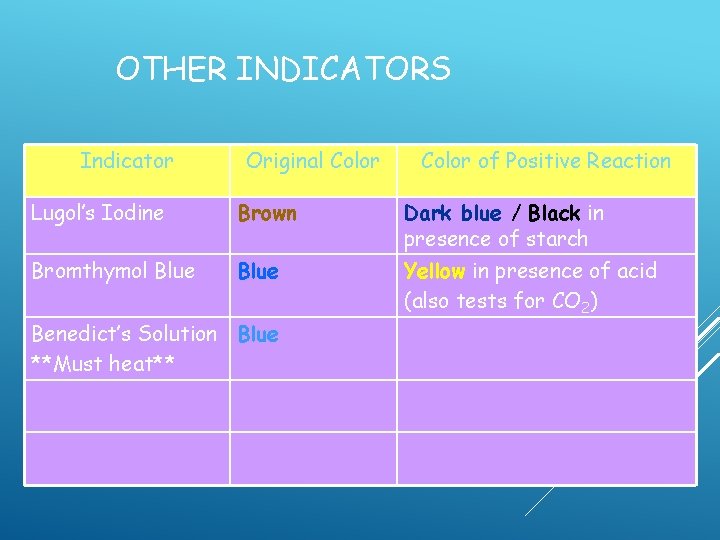
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution Blue **Must heat**
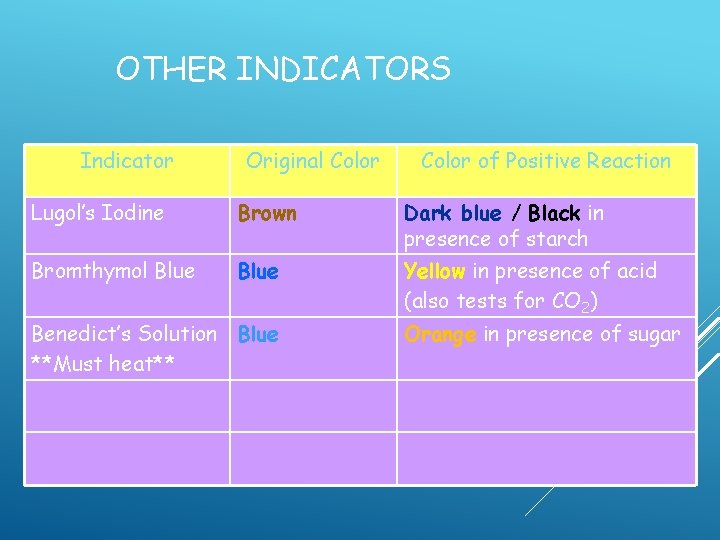
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution Blue **Must heat** Orange in presence of sugar
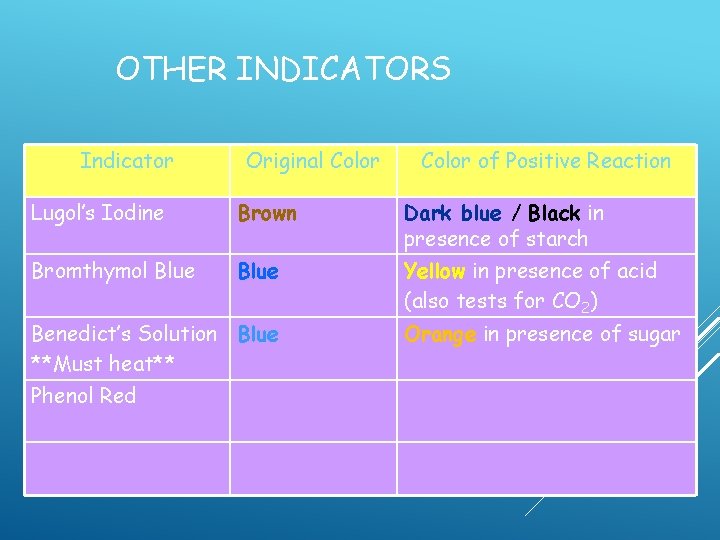
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution Blue **Must heat** Phenol Red Orange in presence of sugar
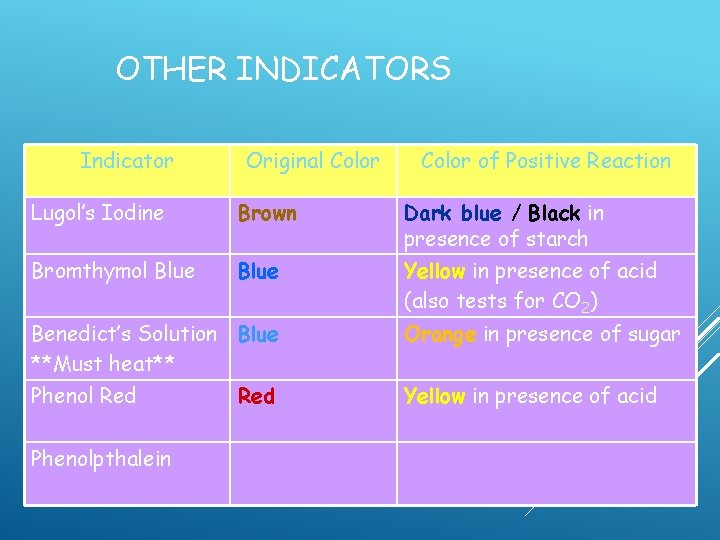
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution Blue **Must heat** Phenol Red Orange in presence of sugar
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution **Must heat** Blue Orange in presence of sugar Phenol Red Yellow in presence of acid Magenta in the presence of base
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution Blue **Must heat** Phenol Red Phenolpthalein Red Orange in presence of sugar Yellow in presence of acid
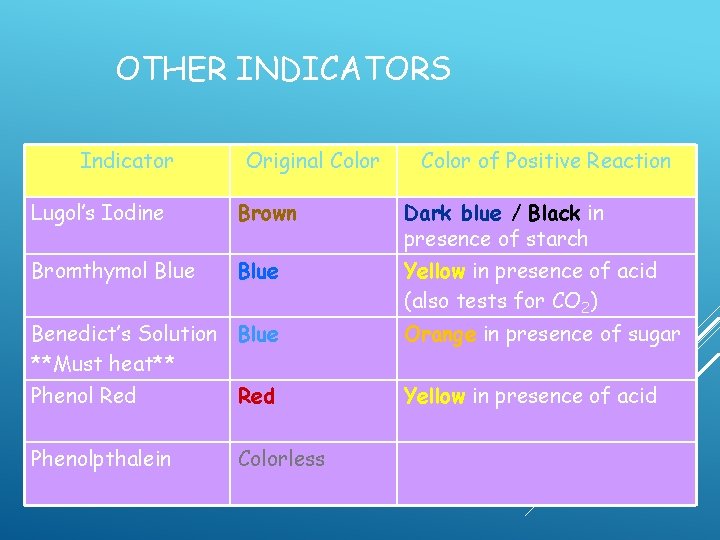
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution Blue **Must heat** Phenol Red Phenolpthalein Colorless Orange in presence of sugar Yellow in presence of acid
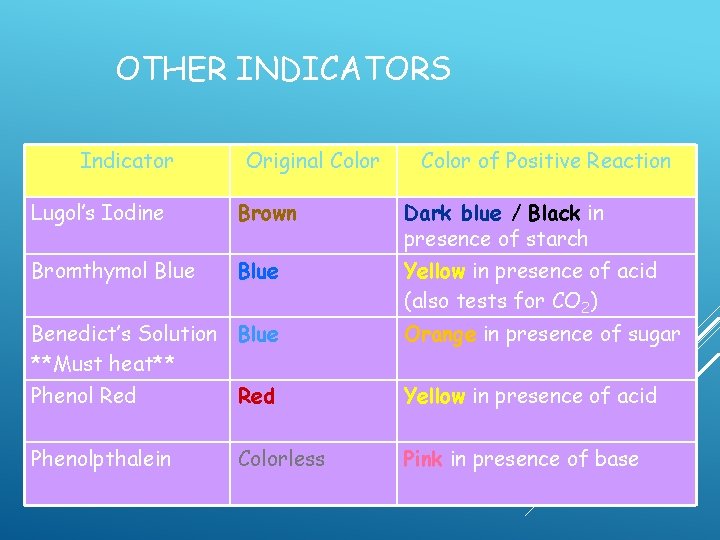
OTHER INDICATORS Indicator Original Color of Positive Reaction Lugol’s Iodine Brown Dark blue / Black in presence of starch Bromthymol Blue Yellow in presence of acid (also tests for CO 2) Benedict’s Solution Blue **Must heat** Orange in presence of sugar Phenol Red Yellow in presence of acid Phenolpthalein Colorless Pink in presence of base
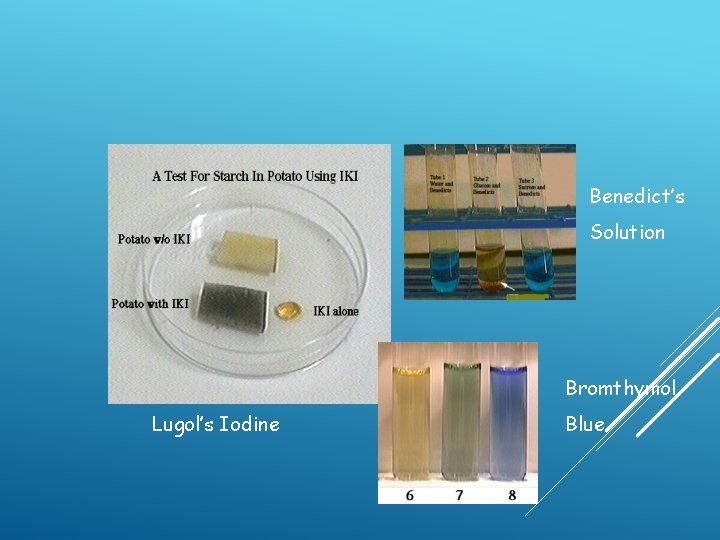
Benedict’s Solution Bromthymol Lugol’s Iodine Blue

CENTRIFUGE Instrument used to separate solutions according to density

Least dense material collects on top Most dense parts settle to the bottom

CHROMATOGRAPHY THIN LAYER CHROMATOGRAPHY (TLC) OR PAPER CHROMATOGRAPHY Procedure used to separate pigments based on density
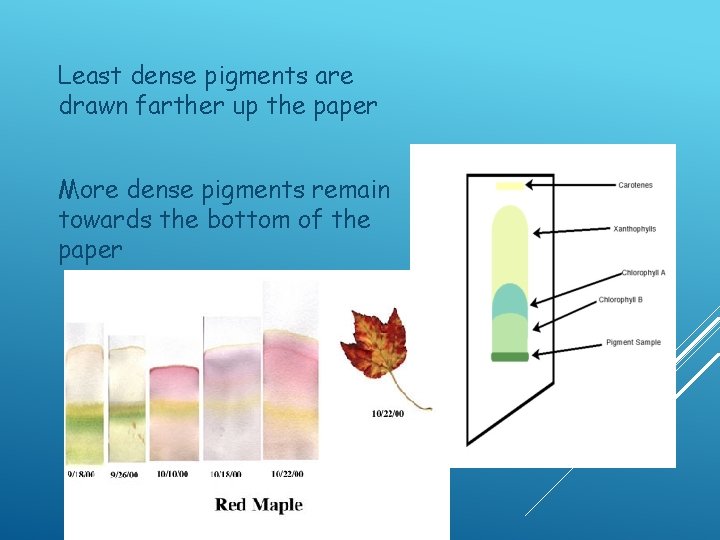
Least dense pigments are drawn farther up the paper More dense pigments remain towards the bottom of the paper
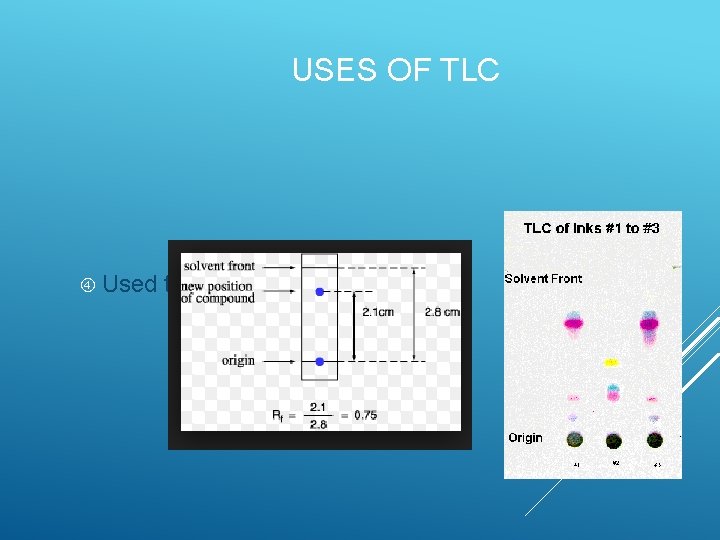
USES OF TLC Used to identify unknown solutions


- Slides: 58