Too little or too much Trace elements and










- Slides: 21

Too little or too much ? : Trace elements and dose response theory

Back to Basics: Elements Remember that all matter is made of elements An element is a substance in its simplest form (an element cannot be separated into simpler substances) For example, the mineral calcite can be broken down to calcium, carbon and oxygen, but these constituent elements can’t be broken down any further (at least at the atomic level) There are over 100 elements, of which 92 occur in nature An atoms of a particular element has a unique number of protons and electrons (number of neutrons can vary, hence the designation of isotopes)

Abundance of Elements Different elements occur in different concentrations in the Earth system We can classify elements on the basis of concentration in nature. For uncommon elements (minor and trace), we usually cite abundance in ppm (parts per million). I part per million would be the equivalent of 1 milligram per 1 kilogram. Major elements: >10, 000 ppm (or about 1% by volume) Minor elements: 1, 000 - 10, 000 ppm Trace elements: <1, 000 ppm on average

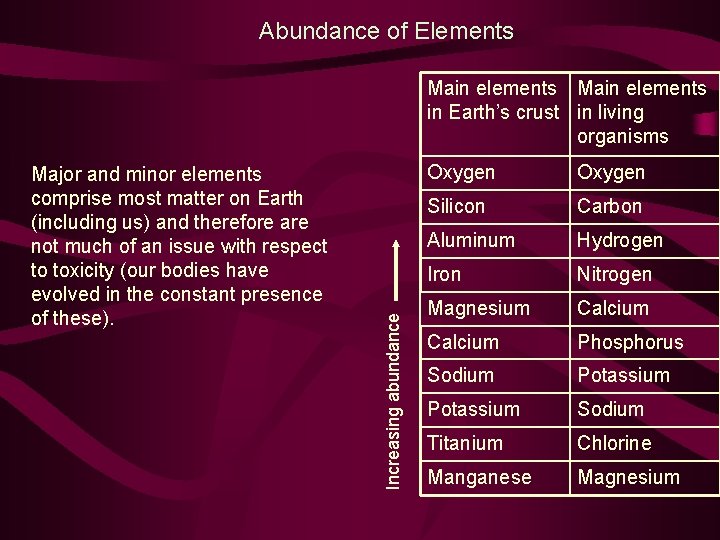
Abundance of Elements Major and minor elements comprise most matter on Earth (including us) and therefore are not much of an issue with respect to toxicity (our bodies have evolved in the constant presence of these). Increasing abundance Main elements in Earth’s crust in living organisms Oxygen Silicon Carbon Aluminum Hydrogen Iron Nitrogen Magnesium Calcium Phosphorus Sodium Potassium Sodium Titanium Chlorine Manganese Magnesium

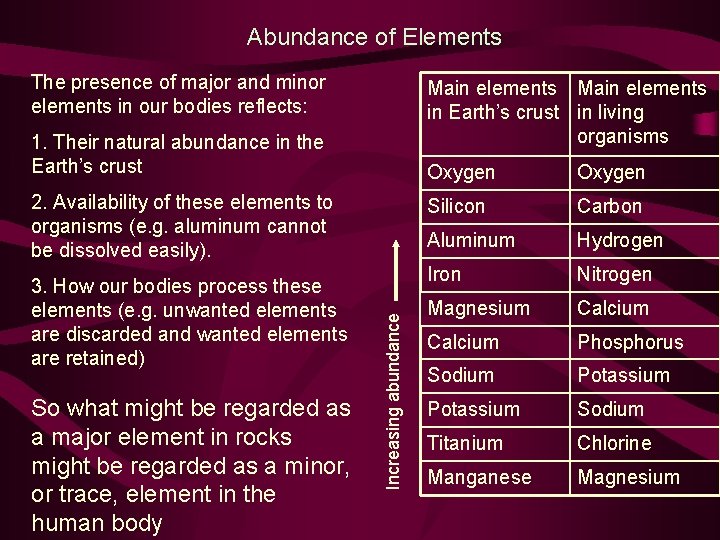
Abundance of Elements The presence of major and minor elements in our bodies reflects: Main elements in Earth’s crust in living organisms 1. Their natural abundance in the Earth’s crust 3. How our bodies process these elements (e. g. unwanted elements are discarded and wanted elements are retained) So what might be regarded as a major element in rocks might be regarded as a minor, or trace, element in the human body Increasing abundance 2. Availability of these elements to organisms (e. g. aluminum cannot be dissolved easily). Oxygen Silicon Carbon Aluminum Hydrogen Iron Nitrogen Magnesium Calcium Phosphorus Sodium Potassium Sodium Titanium Chlorine Manganese Magnesium

Concentration of Elements in the Body Certain tissues of the body accumulate certain elements in higher concentrations, partly due to the role of tissues in the functioning of the human body. For example, fluorine (as fluoride) is incorporated into bones and teeth, so health consequences of variations in concentrations are most likely to be noticed in these tissues. Also, strontium can substitute for calcium in bones, so would be expected to affect bone health. Likewise, the thyroid gland uses iodine to make hormones, so variations in iodine concentration would be expected to be most apparent in the thyroid gland.

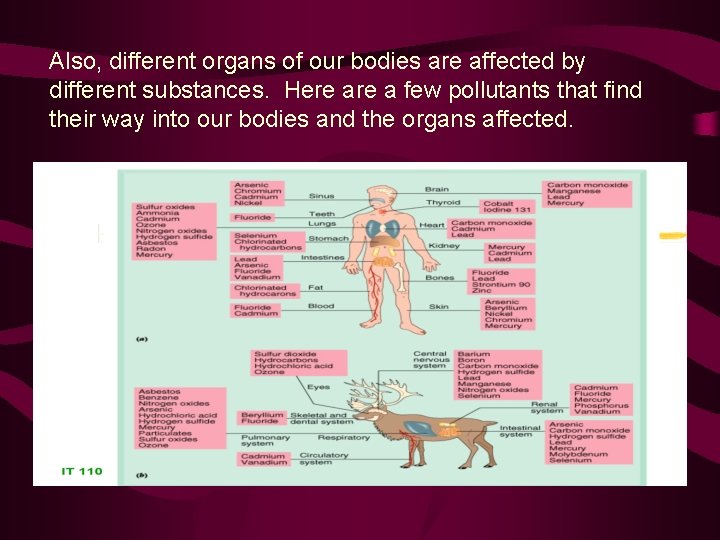
Also, different organs of our bodies are affected by different substances. Here a few pollutants that find their way into our bodies and the organs affected.

Elements in the Earth and in Our Bodies Trace elements are somewhat of a different matter. We have evolved to cope with extremely small quantities of these (and to use some for biological processes). But…slight variations in the abundance of trace elements in our bodies can have profound effects on health. To assess the effects of trace element concentrations on human health, we talk about dose response. Dose response can be depicted in different ways (as we will see), but basically refers to the health response to different doses of a particular substance.

Importance of Element Concentrations Certain concentrations of elements in our bodies are essential to normal functioning A common misconception is that elements such fluorine, known to be beneficial to dental health in certain concentrations, should be more beneficial in high quantities. But this is not so- too little is bad, but too much can be worse. Deficiency or excess of various elements in our bodies is largely controlled by geological factors- whether they be in the direct vicinity of an individual or in the place where food or water are derived (if imported).

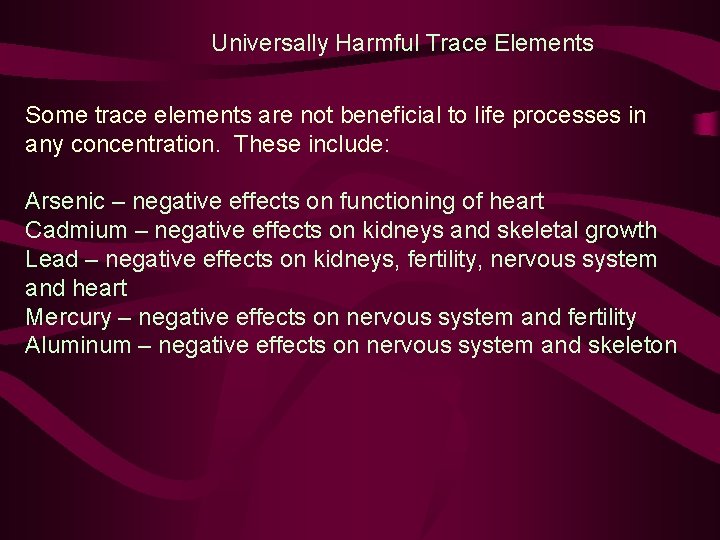
Universally Harmful Trace Elements Some trace elements are not beneficial to life processes in any concentration. These include: Arsenic – negative effects on functioning of heart Cadmium – negative effects on kidneys and skeletal growth Lead – negative effects on kidneys, fertility, nervous system and heart Mercury – negative effects on nervous system and fertility Aluminum – negative effects on nervous system and skeleton

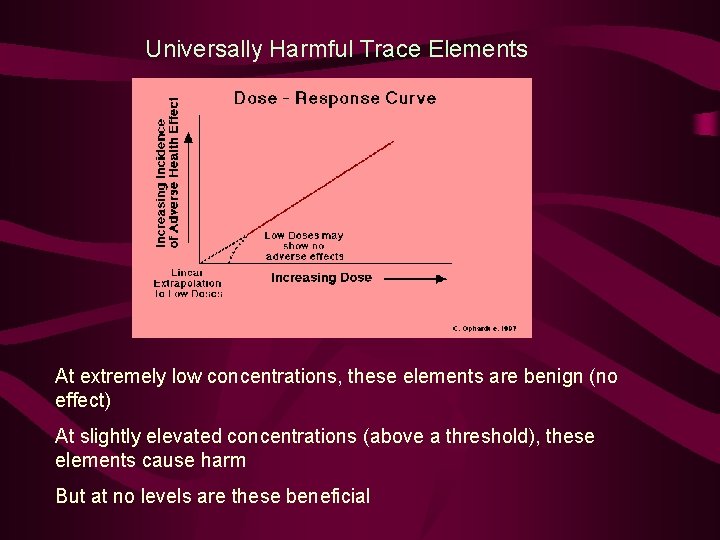
Universally Harmful Trace Elements At extremely low concentrations, these elements are benign (no effect) At slightly elevated concentrations (above a threshold), these elements cause harm But at no levels are these beneficial

Essential Trace Elements Essential trace elements are those elements that are essential for biological processes in low concentrations. These include: Chromium – plays part in metabolism of sugar Cobalt – part of vitamin B-12 Copper – involved in metabolism, production of hemoglobin Fluorine – gives strength to bones and teeth Iodine – used in production of thyroid hormones Iron – essential component of hemoglobin Manganese – involved in bone growth and metabolism Selenium – reduces aging action of free radicals Zinc – important for body growth, and plays part in immune system Note: these are but a few examples of how these elements are used (many have several more functions than indicated)

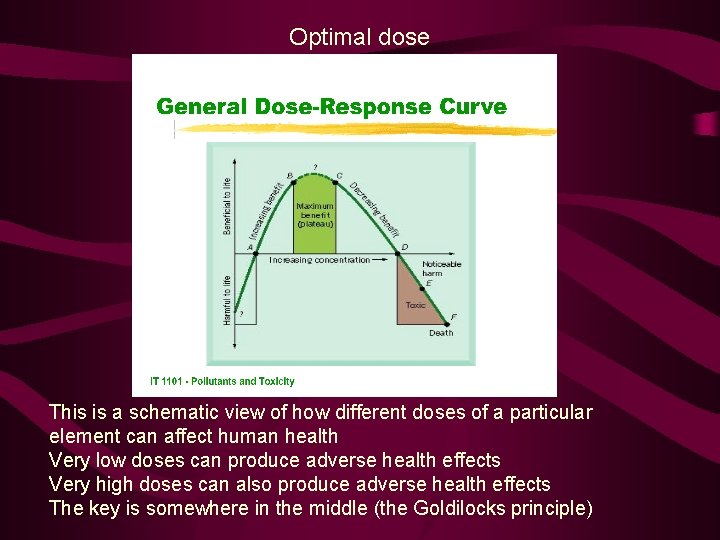
Optimal dose This is a schematic view of how different doses of a particular element can affect human health Very low doses can produce adverse health effects Very high doses can also produce adverse health effects The key is somewhere in the middle (the Goldilocks principle)

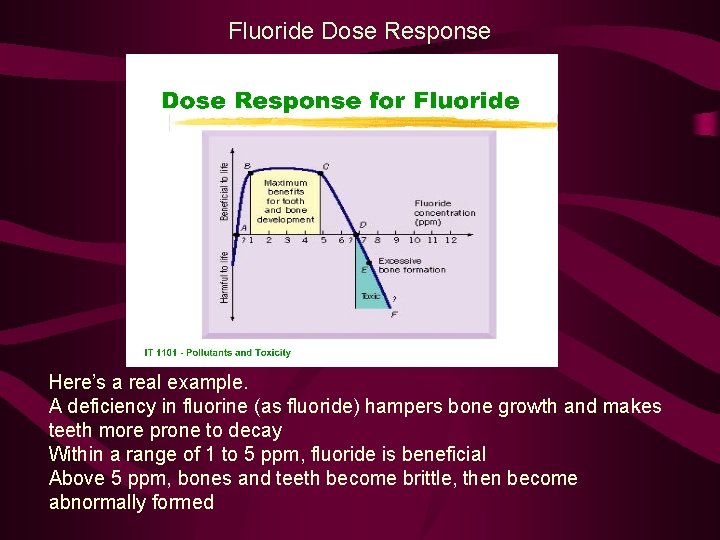
Fluoride Dose Response Here’s a real example. A deficiency in fluorine (as fluoride) hampers bone growth and makes teeth more prone to decay Within a range of 1 to 5 ppm, fluoride is beneficial Above 5 ppm, bones and teeth become brittle, then become abnormally formed

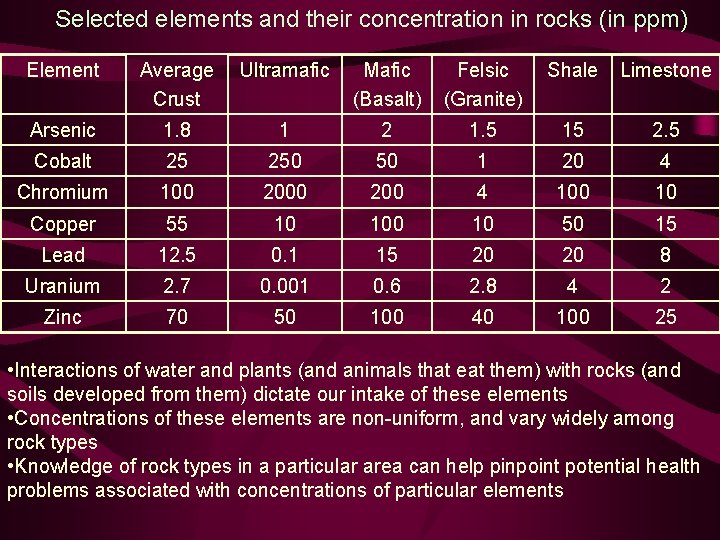
Selected elements and their concentration in rocks (in ppm) Element Average Crust Ultramafic Mafic (Basalt) Felsic (Granite) Shale Limestone Arsenic 1. 8 1 2 1. 5 15 2. 5 Cobalt 25 250 50 1 20 4 Chromium 100 200 4 100 10 Copper 55 10 10 50 15 Lead 12. 5 0. 1 15 20 20 8 Uranium 2. 7 0. 001 0. 6 2. 8 4 2 Zinc 70 50 100 40 100 25 • Interactions of water and plants (and animals that eat them) with rocks (and soils developed from them) dictate our intake of these elements • Concentrations of these elements are non-uniform, and vary widely among rock types • Knowledge of rock types in a particular area can help pinpoint potential health problems associated with concentrations of particular elements

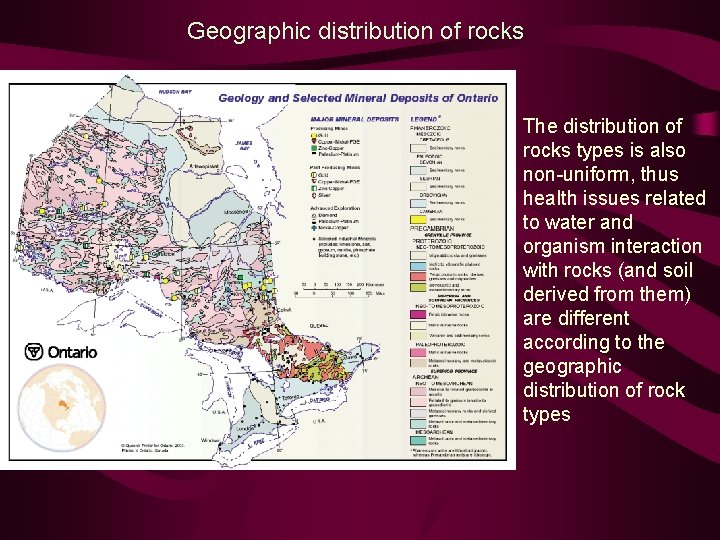
Geographic distribution of rocks The distribution of rocks types is also non-uniform, thus health issues related to water and organism interaction with rocks (and soil derived from them) are different according to the geographic distribution of rock types

Water chemistry On top of this, water chemistry varies Remember, for example, that acidic water tends to dissolved metals more readily than basic water. In turn, water acidity can be linked to pollution derived from industrial and residential areas (largely due to fossil fuel burning). Sudbury - Site of copper/nickel mines - Landscape remains quite barren even though sulphur dioxide emissions have been substantially reduced since the 1960 s (water also remains very acidic) - Area underlain largely by mafic igneous rocks and metamorphic rocks - effect of smelting on wildlife obvious, but what about humans ?

Water movement Plus, water interacts with rocks both at, and below, the Earth’s surface (in vapour and liquid form), and at different rates in different parts of the Earth.

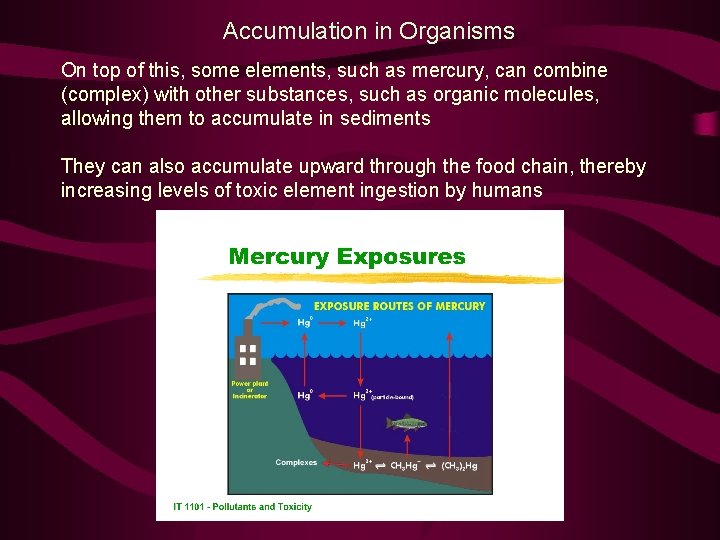
Accumulation in Organisms On top of this, some elements, such as mercury, can combine (complex) with other substances, such as organic molecules, allowing them to accumulate in sediments They can also accumulate upward through the food chain, thereby increasing levels of toxic element ingestion by humans

Can we do it ? So can we even begin to link trace element concentrations and human health ? Yes- but it requires a broad view (this is where geologists come in handy- note that all the major processes are geologically based). In the next couple lectures, we will look at some case studies of the links between trace element concentration and human health.

END OF LECTURE