Tomsk Polytechnic University Tomsk KTH Royal Institute of




- Slides: 9

Tomsk Polytechnic University, Tomsk KTH Royal Institute of Technology, Stockholm Elena Yunda Undergraduate Student, eny@tpu. ru Scientific advisors: Asst. Prof. Anna Godymchuk (TPU) Dr. Gunilla Herting (KTH) Prof. Inger Odnevall Wallinder (KTH) Tomsk- 2014

Ø Background ØGoal/Aim Ø Materials and Methods Ø Results and Discussion Ø Conclusions 2

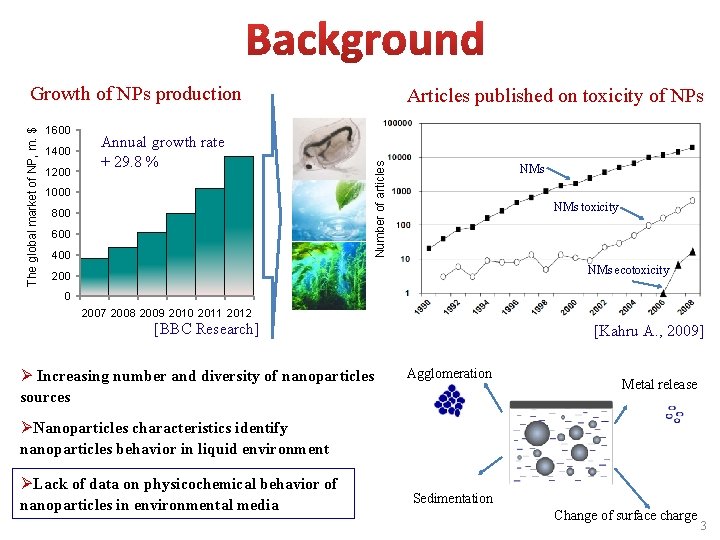
1600 1400 1200 Annual growth rate + 29. 8 % 1000 800 600 400 Articles published on toxicity of NPs Number of articles The global market of NP, m. $ Growth of NPs production NMs toxicity NMs ecotoxicity 200 0 2007 2008 2009 2010 2011 2012 [BBC Research] Ø Increasing number and diversity of nanoparticles sources [Kahru A. , 2009] Agglomeration Metal release ØNanoparticles characteristics identify nanoparticles behavior in liquid environment ØLack of data on physicochemical behavior of nanoparticles in environmental media Sedimentation Change of surface charge 3

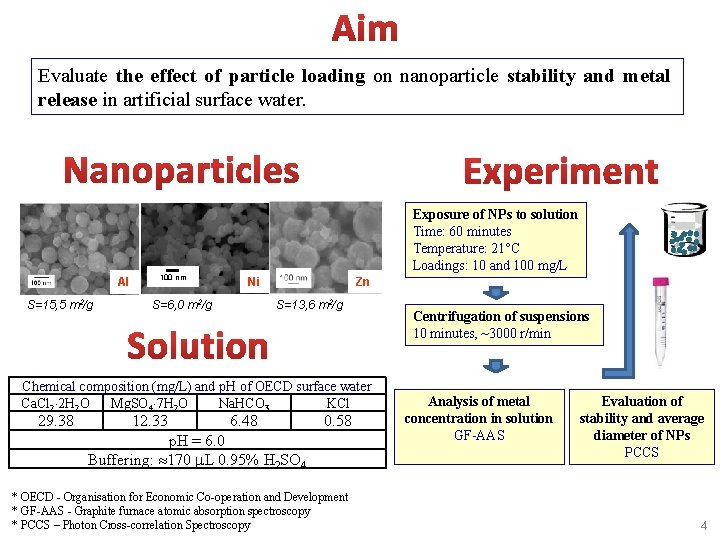
Evaluate the effect of particle loading on nanoparticle stability and metal release in artificial surface water. Al S=15, 5 m 2/g 100 nm Exposure of NPs to solution Time: 60 minutes Temperature: 21°C Loadings: 10 and 100 mg/L Ni S=6, 0 m 2/g Zn S=13, 6 m 2/g Chemical composition (mg/L) and p. H of OECD surface water Ca. Cl 2 2 H 2 O Mg. SO 4 7 H 2 O Na. HCO 3 KCl 29. 38 12. 33 6. 48 0. 58 p. H = 6. 0 Buffering: 170 L 0. 95% H 2 SO 4 * OECD - Organisation for Economic Co-operation and Development * GF-AAS - Graphite furnace atomic absorption spectroscopy * PCCS – Photon Cross-correlation Spectroscopy Centrifugation of suspensions 10 minutes, ~3000 r/min Analysis of metal concentration in solution GF-AAS Evaluation of stability and average diameter of NPs PCCS 4

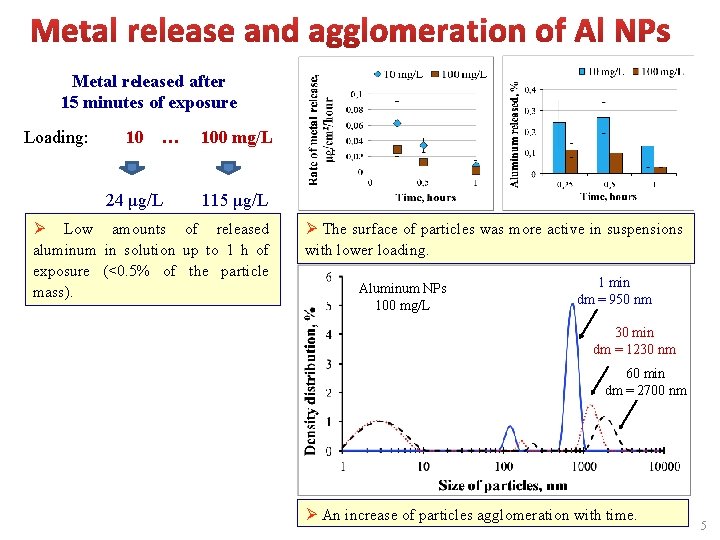
Metal released after 15 minutes of exposure Loading: 10 … 24 μg/L 100 mg/L 115 μg/L Ø Low amounts of released aluminum in solution up to 1 h of exposure (<0. 5% of the particle mass). Ø The surface of particles was more active in suspensions with lower loading. Aluminum NPs 100 mg/L 1 min dm = 950 nm 30 min dm = 1230 nm 60 min dm = 2700 nm Ø An increase of particles agglomeration with time. 5

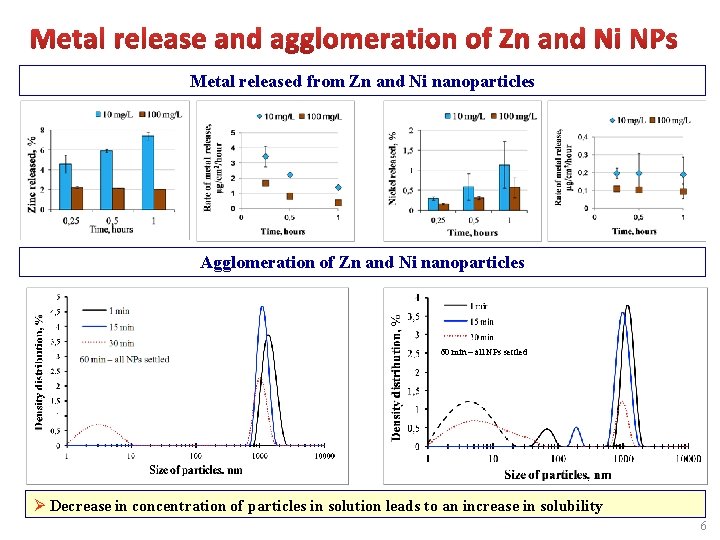
Metal released from Zn and Ni nanoparticles Agglomeration of Zn and Ni nanoparticles 60 min – all NPs settled Ø Decrease in concentration of particles in solution leads to an increase in solubility 6

Zn 10 mg/L Ni 10 mg/L Al 10 mg/L Ø Loading 10 mg/L was below the limits of the instrument under chosen experimental conditions Zn 100 mg/L Ni 100 mg/L Al 100 mg/L Ø Zn and Ni NPs settled after 60 minutes of exposure. Al NPs were relatively stable for 1 hour. 7

Ø Nanoparticles characteristics (size, amount of metal released, stability) changed significantly with time (agglomeration + gradual dissolution + sedimentation). Ø Reducing the loading of nanoparticles in solution in 10 times increased the dissolution rate of Al in 2. 5 times, Zn – in 3. 1 times, Ni – in 2. 2 times. Ø The loading of particles did not affect the kinetics of dissolution. Ø The influence of particles loading on the agglomeration process was not investigated due to the limits of the technique under chosen experimental conditions. 8

Prof. Inger Odnevall Wallinder ingero@kth. se Ass. prof. Anna Godymchuk godymchuk@tpu. ru Dr. Gunilla Herting Ph. D student herting@kth. se Sara Skoglund sarasko@kth. se This work was supported by the scholarship of the President of the Russian Federation for studying abroad and performed at the Division of Surface and Corrosion Science at KTH Royal Institute of Technology, Stockholm, Sweden. 9
 Tomsk polytechnic university rector
Tomsk polytechnic university rector Tomsk polytechnic university rector
Tomsk polytechnic university rector Tpu unipa
Tpu unipa National research tomsk polytechnic university
National research tomsk polytechnic university Kth royal institute of technology notable alumni
Kth royal institute of technology notable alumni Lviv polytechnic university
Lviv polytechnic university Kharkiv polytechnic institute
Kharkiv polytechnic institute Jhenaidah polytechnic institute
Jhenaidah polytechnic institute Dhaka polytechnic institute code
Dhaka polytechnic institute code Worcester polytechnic institute chemical engineering
Worcester polytechnic institute chemical engineering